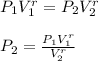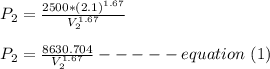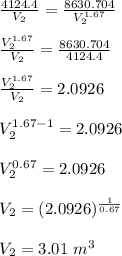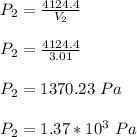Physics, 13.08.2020 01:01 juniorcehand04
Five moles of monatomic ideal gas have initial pressure 2.50 × 103 Pa and initial volume 2.10 m3. While undergoing an adiabatic expansion, the gas does 1680 J of work. What is the final pressure of the gas after the expansion?

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:30
Astudent is given an assignment to demonstrate diffraction. he takes a photograph of a straw in a glass of water. the straw appears bent at the water level. which best describes this example? a) this is a good example of diffraction. b) this is an example of dispersion and not diffraction. c) this is an example of refraction and not diffraction. d) this is an example of reflection and not diffraction.
Answers: 3

Physics, 22.06.2019 12:00
Suppose a wire with a current is pushed upward by a magnetic field. how would the wire move if the direction of the current reversed? explain your answer.
Answers: 1

Physics, 22.06.2019 19:00
Friction removes energy from objects in motion. which statement best describes how this works? a) friction transforms ke into thermal energy b) friction transfers thermal energy to ke c) friction transforms te into pe d) friction transforms pe into ke e) friction transfers ke into pe
Answers: 1

Physics, 22.06.2019 21:10
For the below questions, consider a consumer that consumes two goods, x and z with the following utility function. u with bar on top space equals space x to the power of 1 third end exponent z to the power of 2 over 3 end exponent suppose initial values for income and the prices of goods x and z are y equals 90, p subscript x equals space 10, and p subscript z equals 15 respectively, then the price of good x falls to syntax error from line 1 column 89 to line 1 column 100. unexpected '\'.. what is the magnitude of the total effect
Answers: 3
You know the right answer?
Five moles of monatomic ideal gas have initial pressure 2.50 × 103 Pa and initial volume 2.10 m3. Wh...
Questions


English, 27.07.2019 10:30

Mathematics, 27.07.2019 10:30



Advanced Placement (AP), 27.07.2019 10:30

Physics, 27.07.2019 10:30




English, 27.07.2019 10:30






Geography, 27.07.2019 10:30