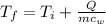Physics, 12.08.2020 18:01 valeriegarcia12
The same heat transfer into identical masses of different substances produces different temperature changes. Calculate the final temperature in degrees Celsius when 1.50 kcal of heat enters 1.50 kg of the following, originally at 15.0°C.(a) water
(b) concrete
(c) steel
(d) mercury

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:50
Select the correct answer from each drop-down menu. compared to its surroundings, the concentration of solutes is low inside a cell. so, the cell is with respect to its surroundings. a particular solute in this cell uses energy for its transport from the cell to its surroundings. this type of transport is called
Answers: 3


Physics, 22.06.2019 21:00
If the specific heat of a metal is 0.850 j/g °c, what is its atomic weight?
Answers: 2

Physics, 22.06.2019 21:00
What do the atoms of elements in the same group have in common? a. they have the same atomic numbers. b. they have the same average atomic masses. c. they have the same number of electron shells. d. they have the same number of electrons in their outermost shells.
Answers: 1
You know the right answer?
The same heat transfer into identical masses of different substances produces different temperature...
Questions




Health, 03.11.2021 07:30

SAT, 03.11.2021 07:30


Mathematics, 03.11.2021 07:30

Computers and Technology, 03.11.2021 07:30




English, 03.11.2021 07:40

Mathematics, 03.11.2021 07:50



Mathematics, 03.11.2021 07:50

French, 03.11.2021 07:50


Mathematics, 03.11.2021 08:00

Mathematics, 03.11.2021 08:00