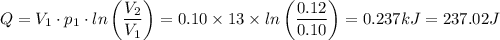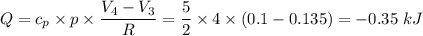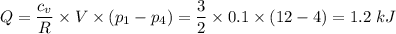Physics, 26.06.2020 17:01 medlinalex
An enclosed amount of nitrogen gas undergoes thermodynamic processes as follows: from an initial state A to a state B to C to D and back to A, as shown in the P-V diagram. Assume that the gas behaves ideally. (a) If the process A-B is isothermal, determine the pressure of the gas in the state B. (b) Calculate the heat transferred to the gas in the process A-B. (c) Calculate the work done on the gas, the heat transferred to the gas and the change in internal energy for the process B-C. (d) What is the change in internal energy of the gas for the entire process, A-B-C-D-A? point A(0.10,13.0) point B(0.12, P) C(0.135, 5.00) D(0.10, 5.00)(V, P)

Answers: 2


Another question on Physics

Physics, 20.06.2019 18:04
Question 9: use your experiences from questions 6, 7, and 8 to you state a general rule for identifying the three declination ranges given the observer's latitude.
Answers: 3

Physics, 22.06.2019 04:40
Steam enters an adiabatic diffuser at 150 kpa and 1208c with a velocity of 550 m/s. determine the minimum velocity that the steam can have at the outlet when the outlet pressure is 300 kpa.
Answers: 3

Physics, 22.06.2019 13:40
Dao makes a table to identify the variables used in the equations for centripetal acceleration. what quantities belong in cells x and y?
Answers: 2

Physics, 22.06.2019 14:30
What conclusion can be made based on the temperature of soil when the light hits the soil at 0°, 45°, and 90° angles in section 2 of the experiment? did your results support your hypothesis? why or why not?
Answers: 1
You know the right answer?
An enclosed amount of nitrogen gas undergoes thermodynamic processes as follows: from an initial sta...
Questions



Mathematics, 07.01.2021 17:50


Mathematics, 07.01.2021 17:50






English, 07.01.2021 17:50

Mathematics, 07.01.2021 17:50




Arts, 07.01.2021 17:50