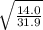Physics, 18.06.2020 18:57 SupremeNerdx
Fill in the blanks for the following: Arigid container of volume 100.0 Liters contains Oxygen gas. It is at room temperature (293 Kelvin), and is at atmospheric pressure (absolute pressure, meaning the gauge pressure is zero). Therefore, the number of moles of Oxygen molecules . Also, the rms-velocity inside is of these Oxygen molecules is most nearly , which is the rms-speed of the Nitrogen molecules just outside the container (the rigid container and its surroundings are in thermal equilibrium).

Answers: 3


Another question on Physics

Physics, 21.06.2019 19:50
An object is dropped from a tower, 400 ft above the ground. the object's height above ground t seconds after the fall is s(t)equals400 minus 16 t squared. determine the velocity and acceleration of the object the moment it reaches the ground. the velocity of the object the moment it reaches the ground is nothing ft/s.
Answers: 1


Physics, 22.06.2019 12:30
Which levels of government establish and implement educational requirements for minors
Answers: 1

Physics, 22.06.2019 16:00
In which of the following is positive work done by a person on a suitcase
Answers: 1
You know the right answer?
Fill in the blanks for the following:
Arigid container of volume 100.0 Liters contains Oxygen gas....
Questions

Mathematics, 18.03.2021 02:00

Computers and Technology, 18.03.2021 02:00







Social Studies, 18.03.2021 02:00


Physics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00

History, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00

Mathematics, 18.03.2021 02:00


Physics, 18.03.2021 02:00


Mathematics, 18.03.2021 02:00

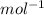

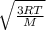
 = 478.6 m/s
= 478.6 m/s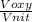 =
= 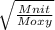
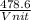 =
=