
Physics, 12.06.2020 21:57 seasmarie75
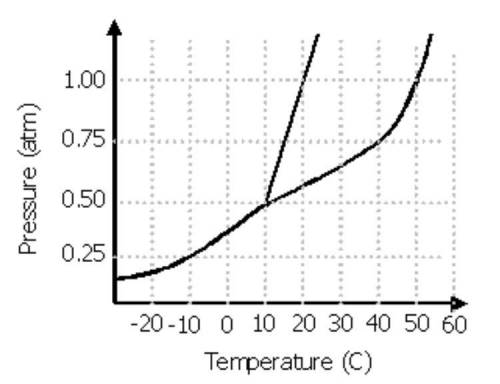
Use the Phase diagram above to answer the questions below.( Remember that standard pressure is 1.00 atm and it is used to find normal phase change temperatures.)
1. In which state/phase of matter is this substance at -10°C and a pressure of 0.50 atm? _
2. What phase change occurs as the substance at 0.75 atm is heated from 0°C to 25°C? _
3. The normal boiling point of a substance is the temperature at standard pressure (1 atm) in which a substance changes from the liquid to the gas/vapor state. What is the normal boiling point of this substance? _
4. The point on the phase diagram where the separation line between liquid and gas ends and the distinction between the liquid phase and gas phase disappears is called the _. At this point, no amount of pressure will cause the substance to condense into a liquid.
5. What is the temperature and pressure of the triple point? _
What phases are in equilibrium at this point? _
6. What phase change occurs as the substance at 0°C has its pressure dropped from 0.50 atm to 0.25 atm? _

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:50
Any color picture tube and electric field exerts a net force of magnitude 1.68x 10^-13 n on an electron the rest mass of an electron is 9.11 x 10^-13 what is the electron acceleration?
Answers: 3

Physics, 22.06.2019 05:30
What do you think car designers do if the damage caused by a crash test is too severe?
Answers: 1


Physics, 23.06.2019 05:20
Derive the formula 2as=v² -u² where the formula have have usual meanings
Answers: 1
You know the right answer?
Use the Phase diagram above to answer the questions below.( Remember that standard pressure is 1.00...
Questions

History, 10.10.2019 16:30






Mathematics, 10.10.2019 16:30


Mathematics, 10.10.2019 16:30




Mathematics, 10.10.2019 16:30


Geography, 10.10.2019 16:30

English, 10.10.2019 16:30



History, 10.10.2019 16:30



