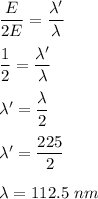When light with a wavelength of 225 nm is incident on a certain metal surface, electrons are ejected with a maximum kinetic energy of 2.98 × 10-19 J. Determine the wavelength (in nm) of light that should be used to double the maximum kinetic energy of the electrons ejected from this surface.

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:50
20. threshold braking in the vehicle's braking system occurs when a. the brake pedal is pushed, heel on floorboard, with full foot pressure. b. the brakes are exerting full braking power, without traction loss. c. the driver begins pushing down the brake pedal, with moderate slowdown. d. the brakes just begin to take hold with a gradual slowdown.
Answers: 3

Physics, 22.06.2019 07:30
Identify the theory that can be used to explain each phenomenon. answers diffraction: wave theory interference: wave theory reflection: both particle and wave theories refraction: both particle and wave theories
Answers: 3

Physics, 22.06.2019 11:30
Which of the following is the phase that results when the moon is on the opposite side of the earth from the sun? a. quarter moon b. crescent moon c. new moon d. full moon
Answers: 1

Physics, 22.06.2019 12:10
Awater slide of length l has a vertical drop of h. abby's mass is m. an average friction force of magnitude f opposes her motion. she starts down the slide at initial speed vi. use work-energy ideas to develop an expression for her speed at the bottom of the slide. then evaluate your result using unit analysis and limiting case analysis. express your answer in terms of the variables h, m, l, vi, f and appropriate constants. vf v f
Answers: 2
You know the right answer?
When light with a wavelength of 225 nm is incident on a certain metal surface, electrons are ejected...
Questions

Geography, 13.03.2021 06:20

Physics, 13.03.2021 06:20


Mathematics, 13.03.2021 06:20


Mathematics, 13.03.2021 06:20


History, 13.03.2021 06:20

English, 13.03.2021 06:20


Mathematics, 13.03.2021 06:20


English, 13.03.2021 06:20

Mathematics, 13.03.2021 06:20

Mathematics, 13.03.2021 06:20


Mathematics, 13.03.2021 06:20


English, 13.03.2021 06:20

Mathematics, 13.03.2021 06:20

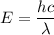
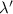 . So,
. So,