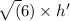Learning Goal:To understand and be able to use the rules for determining allowable orbital angular momentum states. Several numbers are necessary to describe the states available to an electron in the hydrogen atom. The principal quantum number ndetermines the energy of the electron. The orbital quantum number l determines the total angular momentum of the electron, and the magnetic quantum number mldetermines the component of the angular momentum parallel to a specific axis, usually the z axis. For a given principal quantum number n, the orbital quantum number can take integer values ranging from zero to n−1. For a given orbital quantum number l, the magnetic quantum number can take integer values from −l to l. A fourth number, the spin ms, is important for interactions with magnetic fields and counting states. The spin can be either +1/2 or −1/2, independent of the values of the other quantum numbers. The energy of an electron in hydrogen is related to the principal quantum number by En=(−13.60eV)/n2. The orbital angular momentum is related to the orbital quantum number by L=ℏl(l+1)−−−−−−√, and the orbital angular momentum in the z direction is related to the magnetic quantum number by Lz=mlℏ.
A. How many different values of I are possible for an electron with principal quantum number n = 5?
B. How many values of mi are possible for an electron with orbital quantum number I = 3? Express your answer as an integer.
C. The quantum state of a particle can be specified by giving a complete set of quantum numbers (n, l, m1, ms). How many different quantum states are possible if the principal quantum number is n = 3? To find the total number of allowed states, first write down the allowed orbital quantum numbers I, and then write down the number of allowed values of mi for each orbital quantum number. Sum these quantities, and then multiply by 2 to account for the two possible orientations of spin.
D. Is the state n = 3,1 = 3, m1 = -2, ms = 1/2 an allowable state? If not, why not?
a. Yes it is an allowable state.
b. No: The magnetic quantum number cannot be negative.
c. No: The magnetic quantum number must equal the orbital quantum number.
d. No: The orbital quantum number cannot equal the principal quantum number.
e. No: The magnetic quantum number must equal the principal quantum number.
E. What is the maximum angular momentum L max that an electron with principal quantum number n = 3 can have? Express your answer in units of h. (You don't need to enter the h, it is in the units field for you.)

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:40
Sarah and michelle work at a tech company that designs exercise apps. sarah tells michelle she has heard that james, their supervisor, interviewed with a competitor and may be leaving the company, which would open up a management position. what kind of power is sarah exerting?
Answers: 1

Physics, 22.06.2019 09:40
When you jump from an elevated position you usually bend your knees upon reaching the ground. by doing this, you make the time of the impact about 10 times as great as for a stiff-legged landing. in this way the average force your body experiences is a) less than 1/10 as great. b) more than 1/10 as great. c) about 1/10 as great.d) about 10 times as great.
Answers: 1

Physics, 22.06.2019 12:30
Uppose we consider the system of the three capacitors as a single "equivalent" capacitor. given the charges of the three individual capacitors calculated in the previous part, find the total charge qtot for this equivalent capacitor. express your answer in terms of v and c.
Answers: 2

Physics, 22.06.2019 14:30
What distance does a car travel as its speed changes from 0 to 20 m/s in 17 s at constant acceleration?
Answers: 1
You know the right answer?
Learning Goal:To understand and be able to use the rules for determining allowable orbital angular m...
Questions




English, 14.02.2020 04:15