
The rate at which a metal alloy oxidizes in an oxygen-containing atmosphere is a typical example of the practical utility of the Arrhenius equation. For example, the rate of oxidation of a magnesium alloy is represented by a rate constant, k. The value of k at 300°C is 1.05 * 10-8kg/(m4 # s). At 400°C, the value of k rises to 2.95 * 10-4kg/(m4 # s). Calculate the activation energy, Q, for this oxidation process (in units of kJ/mol).

Answers: 3


Another question on Physics

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 19:00
Friction removes energy from objects in motion. which statement best describes how this works? a) friction transforms ke into thermal energy b) friction transfers thermal energy to ke c) friction transforms te into pe d) friction transforms pe into ke e) friction transfers ke into pe
Answers: 1

Physics, 23.06.2019 03:00
It is advised not to hold the thermometer by the bulb while reading it
Answers: 1

Physics, 23.06.2019 03:40
What does every magnet possess? a. iron metal b.strong magnetic field c.repulsion d.north and south poles
Answers: 2
You know the right answer?
The rate at which a metal alloy oxidizes in an oxygen-containing atmosphere is a typical example of...
Questions




SAT, 04.04.2021 14:20

Social Studies, 04.04.2021 14:20

Mathematics, 04.04.2021 14:20

English, 04.04.2021 14:20

Biology, 04.04.2021 14:20

Advanced Placement (AP), 04.04.2021 14:20

Mathematics, 04.04.2021 14:20




English, 04.04.2021 14:20

Physics, 04.04.2021 14:20

Mathematics, 04.04.2021 14:20




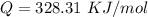
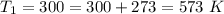
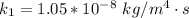
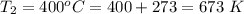
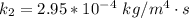
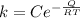
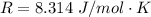
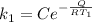
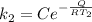
![\frac{k_1 }{k_2} = e^{(\frac{Q}{R} [\frac{1}{\frac{T_2 - 1}{T_1} } ] )}](/tpl/images/0668/9767/c32ab.png)
![ln [\frac{k_1}{k_2} ] = \frac{Q}{R} * [\frac{1}{\frac{T_2 -1}{T_1} } ]](/tpl/images/0668/9767/33b26.png)
![ln [\frac{1.05 *10^{-8}}{2.95 *10^{-4}} ] = \frac{Q}{8.314} * [\frac{1}{\frac{673 -1}{573} } ]](/tpl/images/0668/9767/45b57.png)


