
You put m1 = 1 kg of ice cooled to -20°C into mass m2 = 1 kg of water at 2°C. Both are in a thermally insulated chamber. For water L = 3.33 x 105 J/kg. The specific heat of ice is 2090 J/(kg°C) and of water 4186 J/(kg°C). A. Everything turns to ice at a temperature below 0°C. B. Everything melts and is at a temperature above 0°C. C. There is a mixture of water and ice as the final state. D. The water and ice never reach the same temperature. E. There is not enough information to find the final temperature.

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:30
An electric clothes dryer has a resistance of 8 ohms. it draws 30 a of current. what is the voltage, in volts, of the wall outlet that it is plugged into?
Answers: 2

Physics, 22.06.2019 11:00
What would be the result of an alpha particle coming into a magnetic field? a) the alpha particle will stop moving. b) the alpha particle will reverse its direction. c) the alpha particle will be deflected in a curve path. d) the alpha particle will continue to travel in a straight line.
Answers: 1

Physics, 22.06.2019 18:00
By what primary heat transfer mechanism does the sun warm the earth?
Answers: 1

Physics, 22.06.2019 22:00
If anyonee has done the momentum lab practical in physics can you give me the answers i am s lostt rnn : (
Answers: 2
You know the right answer?
You put m1 = 1 kg of ice cooled to -20°C into mass m2 = 1 kg of water at 2°C. Both are in a thermall...
Questions

Mathematics, 22.02.2021 05:20

Chemistry, 22.02.2021 05:20





Mathematics, 22.02.2021 05:20

Mathematics, 22.02.2021 05:20

Biology, 22.02.2021 05:20





Mathematics, 22.02.2021 05:20






Mathematics, 22.02.2021 05:20

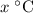 be the final temperature of the mixture. Consider: what are some of the possible values of
be the final temperature of the mixture. Consider: what are some of the possible values of  ? There are three possible outcomes:
? There are three possible outcomes: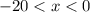 , the final mixture contains only ice.
, the final mixture contains only ice.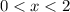 , the final mixture contains only water.
, the final mixture contains only water.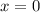 , the final mixture contains both ice and water.Assumption 1
, the final mixture contains both ice and water.Assumption 1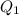 , the energy released when
, the energy released when 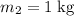 of water is cooled from
of water is cooled from 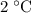 to
to 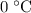 .
.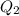 , the energy released when
, the energy released when 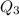 , the energy released when
, the energy released when 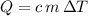 , where
, where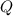 is the energy change due to a change in temperature.
is the energy change due to a change in temperature. is the specific heat capacity.
is the specific heat capacity.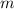 is the mass of the object, and
is the mass of the object, and is the change in temperature. Note that
is the change in temperature. Note that 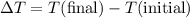 .
.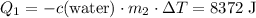 .
.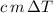 would be negative. Add a minus sign to make sure that the value of
would be negative. Add a minus sign to make sure that the value of 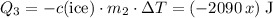 .
.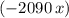 would be positive.
would be positive. the latent heat of fusion of water.
the latent heat of fusion of water.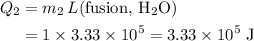 .
.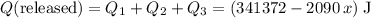 .
.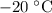 to
to 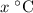 .
.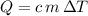 .
.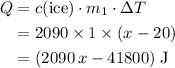 .
.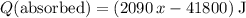 .
.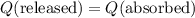 . Therefore:
. Therefore: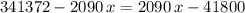 .
.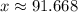 .
. 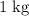 of ice
of ice 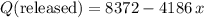 .
.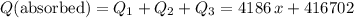 .
.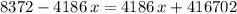
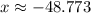 .
. 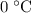 , and the mixture will contain both ice and water.
, and the mixture will contain both ice and water.

