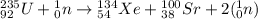Physics, 21.05.2020 23:10 cgonzalez0243
Write a nuclear equation to describe the neutron induced fission of u-235 to form xe-134 and sr-100. Determine how many neutrons are produced in the reaction.

Answers: 1


Another question on Physics

Physics, 22.06.2019 11:10
An isotope undergoes radioactive decay by emitting radiation that has a –1 charge. what other characteristic does the radiation have?
Answers: 3

Physics, 22.06.2019 13:00
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2

Physics, 22.06.2019 22:30
Atennis player used a tennis racket to hit a tennis ball with a mass of 0.25 kg with a force of 5.25 newtons. how does the force the tennis racket exerted on the tennis ball compare with the force the tennis ball exerted on the tennis racket? a. the force of the tennis racket on the tennis ball is equal in magnitude and opposite in direction to the force the tennis ball exerted on the tennis racket. b. the force of the tennis racket on the tennis ball is equal in magnitude and in the same direction as the force the tennis ball exerted on the tennis racket. c. the force of the tennis racket on the tennis ball is less in magnitude and in the same direction as the force the tennis ball exerted on the tennis racket. d. the force of the tennis racket on the tennis ball is greater in magnitude and opposite in direction as the force the tennis ball exerted on the tennis racket.
Answers: 1

Physics, 23.06.2019 04:31
Elements in group viiia (also known as group 18, or the noble gases) have similar properties because they all have a. the same number of electron shells b. full octets c. the same number of valence electrons d. the same number of electrons needed to fill their octet
Answers: 1
You know the right answer?
Write a nuclear equation to describe the neutron induced fission of u-235 to form xe-134 and sr-100....
Questions

Geography, 09.01.2020 10:31

Arts, 09.01.2020 10:31

Mathematics, 09.01.2020 10:31












Mathematics, 09.01.2020 10:31


Mathematics, 09.01.2020 10:31


History, 09.01.2020 10:31

Chemistry, 09.01.2020 10:31