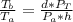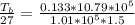Physics, 21.05.2020 04:05 davisnaziyahovz5sk
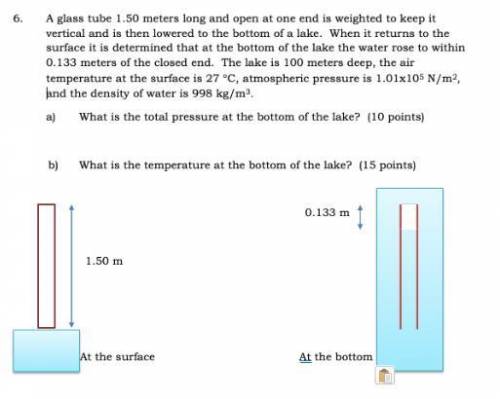
A glass tube 1.50 meters long and open at one end is weighted to keep it vertical and is then lowered to the bottom of a lake. When it returns to the surface it is determined that at the bottom of the lake the water rose to within 0.133 meters of the closed end. The lake is 100 meters deep, the air temperature at the surface is 27 "C, atmospheric pressure is 1.01x10s N/m2, and the density of water is 998 kg/m3. a) What is the total pressure at the bottom of the lake

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:00
Did the proton move into a region of higher potential or lower potential? did the proton move into a region of higher potential or lower potential? because the proton is a negative charge and it accelerates as it travels, it must be moving from a region of higher potential to a region of lower potential.because the proton is a negative charge and it accelerates as it travels, it must be moving from a region of lower potential to a region of higher potential.because the proton is a positive charge and it slows down as it travels, it must be moving from a region of higher potential to a region of lower potential.because the proton is a positive charge and it slows down as it travels, it must be moving from a region of lower potential to a region of higher potential.request answerpart bwhat was the potential difference that stopped the proton? express your answer with the appropriate units.î”v î” v = nothingnothingrequest answerpart cwhat was the initial kinetic energy of the proton, in electron volts? express your answer in electron volts.ki k i = nothing ev request answerprovide feedback
Answers: 2

Physics, 22.06.2019 02:00
What is created when solids,liquids,an gases mix with one another
Answers: 1

Physics, 22.06.2019 07:10
1. how much energy is needed to raise the temperature of 40.0 g of argon from 25c to 40c? the specific heat capacity of argon is 0.520 j/(g·k) 2a. 23.0 ml of 0.100 m hcl (standard) are added from a buret to neutralize 50.0 ml of an unknown basic solution. 2b. if the oh- produced in the previous reaction came from ca(oh)2, then what is the molarity of the ca(oh)2? 3.calculate the new freezing-point of a solution when 60.5 grams of cacl2 solute is dissolved in 0.612 kg of water. 4. what is the maximum number of moles of alcl3 that can be produced from 5.0 mol al and 6.0 mol cl2? 5. a sample of oxygen gas has a volume of 150 ml when its pressure is 0.923 atm. if the pressure is increased to 0.987 atm and the temperature remains constant, what will the new volume be? 6. nitrogen gas in a closed container at a temperature of 100.0 oc and 3.0 atm is heated to 300 oc. what is the pressure of the gas at the higher temperature?
Answers: 3

Physics, 22.06.2019 10:20
Assume that a person skiing high in the mountains at an altitude of h = 15100 ft takes in the same volume of air with each breath as she does while walking at sea level. determine the ratio of the mass of oxygen inhaled for each breath at this high altitude compared to that at sea level. assume that the air composition (i.e. % of air that is oxygen) is the same at sea level as it is at 15100 ft.
Answers: 2
You know the right answer?
A glass tube 1.50 meters long and open at one end is weighted to keep it vertical and is then lowere...
Questions



Health, 05.10.2019 15:00

History, 05.10.2019 15:00

English, 05.10.2019 15:00


Mathematics, 05.10.2019 15:00




Social Studies, 05.10.2019 15:00

Geography, 05.10.2019 15:00


Mathematics, 05.10.2019 15:00


Mathematics, 05.10.2019 15:00


Mathematics, 05.10.2019 15:00


Business, 05.10.2019 15:00

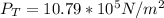
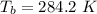
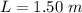
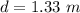
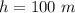
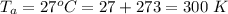
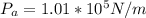
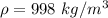
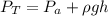
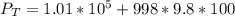
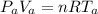
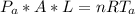
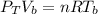
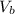 is the volume of the glass tube not covered by water at bottom
is the volume of the glass tube not covered by water at bottom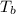 i the temperature at the bottom
i the temperature at the bottom