
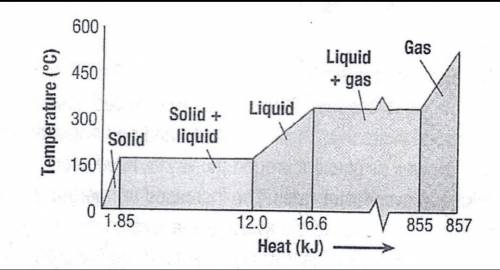
The graph below shows the change in average kinetic energy, as represented by temperature, of a 23 g sample of a substance as energy is added to the substance as heat.
What is the specific heat capacity of the solid?
A. 1.85 × 10³ J/kg•°C
B. 4.0 × 10² J/kg•°C
C. 5.0 × 10² J/kg•°C
D. 1.1 × 10³ J/kg•°C

Answers: 1


Another question on Physics

Physics, 21.06.2019 14:40
If you go to a nutrition store and buy a supplement, you can count on the fact that it is pure and safe because supplements are regulated, controlled, and inspected by the fda.
Answers: 1


Physics, 21.06.2019 22:50
If the temperature were raised very high, classically what would we expect the heat capacity per object to be for this one-dimensional system? give a numerical value. chigh t = __ j/k/object (one reason for the discrepancy is that the high-temperature limit assumes that the number of oscillators is large (n > > 1), which is not the case in this tiny system.)
Answers: 2

You know the right answer?
The graph below shows the change in average kinetic energy, as represented by temperature, of a 23 g...
Questions

English, 14.04.2020 21:38

History, 14.04.2020 21:38











Mathematics, 14.04.2020 21:38

Mathematics, 14.04.2020 21:38

History, 14.04.2020 21:38


Mathematics, 14.04.2020 21:38





