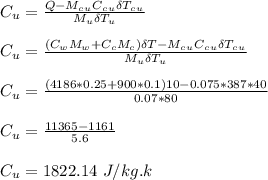Physics, 06.05.2020 16:01 brandonleestewovilgw
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal equilibrium at 10.0°C. Two metallic blocks are placed into the water. One is a 75.0-g piece of copper at 60.0°C. The other has a mass of 70.0 g and is originally at a temperature of 100°C. The entire system stabilizes at a final temperature of 20.0°C.
Determine the specific heat of the unknown sample.

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:00
Six solutions are made, each with a y concentration of 1.0 μm and varying concentrations of ab as shown below. based on the concentrations, rank the solutions in decreasing order of reaction rate.
Answers: 2

Physics, 22.06.2019 12:30
Urgent pls a. coal consumption levels off and remains flat. b. petroleum, natural gas, and renewables show an increase in consumption c. more nonrenewable resources continued to be consumed than renewable. d. there is little projected increase in nuclear energy use. e. carbon dioxide emissions are projected to decline as we approach 2040. global energy consumption is defined as the total energy used by an individual or organizations from around the world. use the graph above to analyze the projected energy consumption from now until 2040. which statements in the prompt apply? a) a, b, d b) b, c, d c) a, c, d d) a, b, c, d
Answers: 1


Physics, 22.06.2019 17:00
Since the energy was not raising the temperature of the water during these segments, what was the energy used to do instead? your answer must include an explanation of what is happening to the molecules.
Answers: 3
You know the right answer?
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are...
Questions

History, 01.11.2019 02:31






English, 01.11.2019 02:31

History, 01.11.2019 02:31



Mathematics, 01.11.2019 02:31

History, 01.11.2019 02:31

English, 01.11.2019 02:31



History, 01.11.2019 02:31



Mathematics, 01.11.2019 02:31

Mathematics, 01.11.2019 02:31

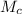 = 100 g
= 100 g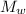 = 250 g
= 250 g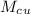 = 75 g
= 75 g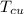 = 60.0°C
= 60.0°C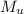 = 70.0 g
= 70.0 g 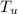 = 100°C.
= 100°C.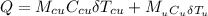
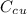 is the specific heat capacity of copper
is the specific heat capacity of copper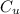 is the specific heat capacity of unknown sample
is the specific heat capacity of unknown sample