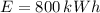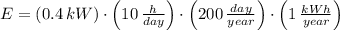Physics, 06.05.2020 03:17 kmontanab00
Ceiling-mounted fluorescent lighting fixtures with total power equal to 400 W are used to produce an average illumination level of 600 lux on student desks in a particular classroom. If the classroom lights are on 10 hours a day for 200 days per year, calculate the annual energy used for lighting in this classroom.
A 800 kWh
B 2 kWh
C 2,000 Wh
D 2,000 kWh
E 800,000 kWh
F 600 kWh

Answers: 3


Another question on Physics

Physics, 21.06.2019 19:20
How would you place two electrons on a sphere of radius r so that the electrostatic potential energy is minimized. how would you place three electrons to solve the same problem.
Answers: 1

Physics, 22.06.2019 00:10
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 01:00
An object is 10 cm from the mirror, its height is 1 cm and the focal length is 5 cm. what is the image height? (indicate the object orientation by including the + or - sign with the answer.) hi = cm +1 -1 +10 -10
Answers: 3

Physics, 22.06.2019 07:10
Road users moving into your lane, brake lights, and abrupt changes in road surface are a. rare at night b. indicators of potential hazards c. not worth worrying about before you reach them d. no problem for experienced drivers
Answers: 1
You know the right answer?
Ceiling-mounted fluorescent lighting fixtures with total power equal to 400 W are used to produce an...
Questions



Mathematics, 03.01.2020 00:31

Physics, 03.01.2020 00:31


Computers and Technology, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31

History, 03.01.2020 00:31


Biology, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31

Physics, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31



Mathematics, 03.01.2020 00:31


Social Studies, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31