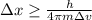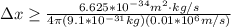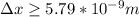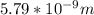German physicist Werner Heisenberg related the uncertainty of an object's position ( Δ x ) to the uncertainty in its velocity ( Δ v ) Δ x ≥ h 4 π m Δ v where h is Planck's constant and m is the mass of the object. The mass of an electron is 9.11 × 10 − 31 kg. What is the uncertainty in the position of an electron moving at 2.00 × 10 6 m/s with an uncertainty of Δ v = 0.01 × 10 6 m/s ?

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:30
Which feature of a heating curve indicates a change ofn state
Answers: 1

Physics, 22.06.2019 02:30
Eddy whose mass is 55kg climbs up the 1.50 meter high stairs in 2s.calculate eddy’s power rating
Answers: 1


Physics, 22.06.2019 20:10
Consider two less-than-desirable options. in the first you are driving 30 mph and crash head-on into an identical car also going 30 mph. in the second option you are driving 30 mph and crash head-on into a stationary brick wall. in neither case does your car bounce off the thing it hits, and the collision time is the same in both cases. which of these two situations would result in the greatest impact force?
Answers: 1
You know the right answer?
German physicist Werner Heisenberg related the uncertainty of an object's position ( Δ x ) to the un...
Questions



Mathematics, 28.05.2021 02:00



Mathematics, 28.05.2021 02:00

English, 28.05.2021 02:00




Mathematics, 28.05.2021 02:00

Mathematics, 28.05.2021 02:00

Arts, 28.05.2021 02:00

Spanish, 28.05.2021 02:00





Mathematics, 28.05.2021 02:00


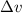 = Uncertainty in velocity of object
= Uncertainty in velocity of object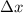 = Uncertainty in position of object
= Uncertainty in position of object