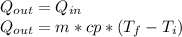reaches an equilibrium temperature of 31.1°C.

Physics, 17.04.2020 06:47 johnisawesome999
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
In an attempt to cool the liquid, which has a
mass of 177 g. 110 g of ice at 0.0°C is added.
At the time at which the temperature of the
tea is 29.1°C, find the mass of the remaining ice in the jar. The specific heat of water is 4186 J/kg.°C. Assume the specific heat capacity of the tea to be that of pure liquid water. Answer in units of g.

Answers: 2


Another question on Physics


Physics, 22.06.2019 13:20
Which of the following is the main energy source for the sun? a. fission of hydrogen b. fusion of hydrogen c. fusion of helium d. fission of iron
Answers: 2

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 22:00
Notice how they put future which of the clouds shown would indicate a possible future rain storm? a) b) c) d)
Answers: 1
You know the right answer?
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
reaches an equilibrium temperature of 31.1°C.
Questions

English, 05.10.2019 16:30

History, 05.10.2019 16:30



Business, 05.10.2019 16:30



History, 05.10.2019 16:30

Mathematics, 05.10.2019 16:30


Chemistry, 05.10.2019 16:30



Spanish, 05.10.2019 16:30

History, 05.10.2019 16:30


Mathematics, 05.10.2019 16:30