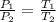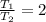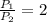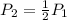Physics, 14.04.2020 23:21 potatogirl6811
Considering what you now know about Gay-Lussac’s law, make a prediction based on the following situation: What would happen to the pressure of a gas inside a sealed bottle, if the bottle was cooled to half of its original temperature? Explain your thoughts.

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:10
The graph below represents the work done by a student while running up flights of stairs. determine the student's power output after 10 seconds: 1600 j/s 8000 j/s 160 j/s 800 j/s
Answers: 3

Physics, 21.06.2019 16:30
Which of the following is not a part of a wave? question 2 options: a: frequency b: refraction c: wavelength d: amplitude (whoever answers correctly first gets brainiest! ) ~kayla
Answers: 2

Physics, 21.06.2019 16:40
Three point charges, two positive and one negative, each having a magnitude of 20 μc are placed at the vertices of an equilateral triangle (30 cm on a side). what is the magnitude of the electrostatic force on the negative charge
Answers: 3

Physics, 21.06.2019 23:00
We want to calculate the total metabolic heat generated by a singing canary taking into account heat transfer by radiation, convection and exhaling air. the air temperature is 20 oc, canary’s body internal and surface temperature is 33oc, external body surface convective heat transfer coefficient is 25.2 w/m2 .k, temperature difference between the inhaled and exhaled air is 4.3 oc, the ventilation rate is 0.74 cc of air per second, specific heat of air is 1.0066 kj/kg.k and density of air is 1.16 kg/m3 . assume the canary’s body to be a cylinder with 7 cm diameter and 9 cm length, and heat exchange is from the side as well as the top and bottom of cylinder. calculate 1) the net rate of heat lost by radiation, assuming heat gained by the bird through radiation from the surroundings is 11.5 w; 2) rate of heat transferred by convection to the surrounding air; 3) rate of heat transferred in the exhaling air without considering any internal evaporation; 4) total metabolic power.
Answers: 2
You know the right answer?
Considering what you now know about Gay-Lussac’s law, make a prediction based on the following situa...
Questions




Mathematics, 04.07.2020 14:01



Computers and Technology, 04.07.2020 14:01





Computers and Technology, 04.07.2020 14:01





Mathematics, 04.07.2020 14:01


Business, 04.07.2020 14:01