
Physics, 07.04.2020 21:27 ellie55991
A galvanic cell based on these half-reactions is set up under standard conditions where each solutions is 1.00 L and each electrode weighs exactly 100.0 g. How much will the Cd electrode weigh when the non-standard potential of the cell is 0.03305 V?

Answers: 2


Another question on Physics

Physics, 22.06.2019 04:10
You will be galileo perform the experiment to determine if objects with different mass fall at the same, or different, rates in the air and in a vacuum. (refer to the walk-through video to locate the online lab within the online textbook).
Answers: 2

Physics, 22.06.2019 23:30
A6.0-kilogram cart initially traveling at 4.0 meters per second east accelerates uniformly at 0.50 meter per second squared east for 3.0 seconds. what is the speed of the cart at the end of this 3.0 second interval? (1) 1.5 m/s (3) 3.0 m/s (2) 5.5 m/s (4) 7.0 m/s
Answers: 1

Physics, 23.06.2019 00:00
A15,000 kg rocket traveling at +230 m/s turns on its engines. over a 6.0 s period it burns 1,000 kg of fuel. an observer on the ground measures the velocity of the expelled gases to be −1,200 m/s.
Answers: 3

Physics, 23.06.2019 01:30
Sally turns on her cellular telephone to speak to her friend who is located thousands of miles away. which of the following best describes how such a telephone is able to transmit and receive information? a. the cellular telephone transmits, receives, and encodes information using only sound waves. b. the cellular telephone transmits, receives, and encodes information using only electromagnetic waves. c. the cellular telephone transmits information by electromagnetic waves to a receiver which then encodes them and produces sound. d. the cellular telephone transmits information by sound waves to a receiver which then encodes them and produces electromagnetic waves.
Answers: 2
You know the right answer?
A galvanic cell based on these half-reactions is set up under standard conditions where each solutio...
Questions


Mathematics, 06.07.2019 22:00


History, 06.07.2019 22:00










Mathematics, 06.07.2019 22:00

History, 06.07.2019 22:00




Health, 06.07.2019 22:00


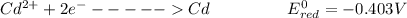
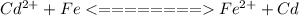
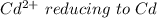 is higher than the potential of
is higher than the potential of 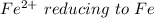 so the the Fe would be oxidized and
so the the Fe would be oxidized and 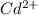 would be reduced
would be reduced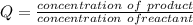
![= \frac{[Fe^{2+}[Cd]]}{[Cd^{2+}][Fe]}](/tpl/images/0587/3820/896ae.png)
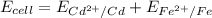
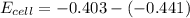
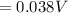
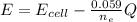
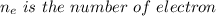
![E = E_{cell} - \frac{0.059}{n_e} \frac{[Fe^{2+}[Cd]]}{[Cd^{2+}][Fe]}](/tpl/images/0587/3820/44521.png)
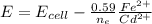
![E= E_{cell} - \frac{0.059}{n_e} [\frac{1}{Cd^{2+}} ]](/tpl/images/0587/3820/d4513.png)
![[Cd^{2+}] =\frac{1}{e^{[\frac{0.03305- 0.038}{\frac{0.059 }{2} }] }}](/tpl/images/0587/3820/4aa4f.png)
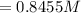
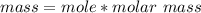
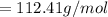
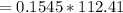
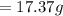
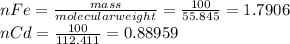
![E=0.038-\frac{0.0592}{2} log\frac{[Fe^{2+}] }{[Cd^{2+}] } \\0.03305=0.038-0.0296log\frac{1.7906+x}{0.88959-x}](/tpl/images/0587/3820/10740.png)


