PART ONE
If it has enough kinetic energy, a molecule
at the surface of the Earth c...

PART ONE
If it has enough kinetic energy, a molecule
at the surface of the Earth can escape the Earth’s gravitation.
The acceleration of gravity is 9.8 m/s^2
,and the Boltzmanns’ constant is 1.38066 ×
10^−23 J/K. Using energy conservation, determine the minimum kinetic energy needed to escape in terms of the mass of the molecule m, the
free-fall acceleration at the surface g, and the
radius of the Earth R. (IMAGE)
PART TWO
Calculate the temperature for which the minimum escape energy is 15 times the average
kinetic energy of an oxygen molecule.
Answer in units of K.
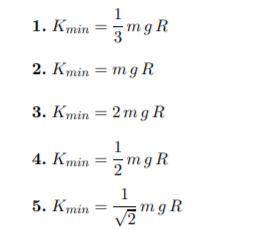

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:50
What is the type of the reaction below? bacl2 + h2so4 → 2 hci + baso4 double replacement synthesis single replacement decomposition
Answers: 1

Physics, 22.06.2019 01:30
Ajet plane has a sound level of 120 db at a distance of 60 m. what is the q23. sound level at a distance of 6.0 km? (60)2 (6000)? om solution 80db db, hint: 16x assume ultrasound waves travel through the body of an animal at 1540 q24. m/s. if a 30,000 hz signal were reflected off a portion of a heart which was moving toward the source at 3 m/s, what frequency signal would return to the stationary source? solution 30.117hz
Answers: 3

Physics, 22.06.2019 07:10
1. how much energy is needed to raise the temperature of 40.0 g of argon from 25c to 40c? the specific heat capacity of argon is 0.520 j/(g·k) 2a. 23.0 ml of 0.100 m hcl (standard) are added from a buret to neutralize 50.0 ml of an unknown basic solution. 2b. if the oh- produced in the previous reaction came from ca(oh)2, then what is the molarity of the ca(oh)2? 3.calculate the new freezing-point of a solution when 60.5 grams of cacl2 solute is dissolved in 0.612 kg of water. 4. what is the maximum number of moles of alcl3 that can be produced from 5.0 mol al and 6.0 mol cl2? 5. a sample of oxygen gas has a volume of 150 ml when its pressure is 0.923 atm. if the pressure is increased to 0.987 atm and the temperature remains constant, what will the new volume be? 6. nitrogen gas in a closed container at a temperature of 100.0 oc and 3.0 atm is heated to 300 oc. what is the pressure of the gas at the higher temperature?
Answers: 3

Physics, 22.06.2019 08:30
Does anyone know how to solve this problem? i really need . i made an attempt but i just cant get it. a metal rod is 25.000 cm long at 25.0 degrees celsius. when heated to 102.0 degrees celsius, it is 25.054 cm long. what is the coefficient of linear expansion for this metal.
Answers: 3
You know the right answer?
Questions

Mathematics, 31.08.2021 01:20

Advanced Placement (AP), 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20


Biology, 31.08.2021 01:20

History, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20



Mathematics, 31.08.2021 01:20


World Languages, 31.08.2021 01:20

Mathematics, 31.08.2021 01:20



Mathematics, 31.08.2021 01:30



