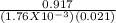Physics, 31.03.2020 23:35 kayonapretty14p45995
Gaseous carbon dioxide is partially decomposed according to the following equation. An initial pressure of 1.00 atm of CO2 is placed in a closed container at 2500 K, and 2.1 % of the molecules decompose. Determine the equilibrium constant Kp at this temperature

Answers: 1


Another question on Physics


Physics, 22.06.2019 12:50
Assume you measured the mass of the cart to be (500 ± 1) g and the mass of the additional mass you put on the cart to be (500 ± 1) g as well. since the scale you are using in the lab cannot measure objects heavier than 600g you will have to sum up individual pieces and propagate the error. so what would be the mass and the standard error of the cart and the mass
Answers: 3

Physics, 22.06.2019 13:00
At a certain instant after jumping from the airplane a, a skydiver b is in the position shown and has reached a terminal (constant) speed vb = 52 m/s. the airplane has the same constant speed va = 52 m/s, and after a period of level flight is just beginning to follow the circular path shown of radius ρa = 2330 m. (a) determine the velocity and acceleration of the airplane relative to the skydiver. (b) determine the time rate of change of the speed vr of the airplane and the radius of curvature ρr of its path, both as observed by the nonrotating skydiver.
Answers: 3

Physics, 22.06.2019 16:20
How does a circuit breaker protect a refrigerator? a. when the current is too high, a metal strip in the fuse melts and opens the circuit. b. when the resistance is too high , a re-settable which opens a circuit c. when the current is too high , a re-settable switch opens the circuit d. when the resistance is too high a metal strip in the fuse melts and opens the circuit
Answers: 2
You know the right answer?
Gaseous carbon dioxide is partially decomposed according to the following equation. An initial press...
Questions

Biology, 01.10.2019 23:00


Chemistry, 01.10.2019 23:00



Biology, 01.10.2019 23:00


Computers and Technology, 01.10.2019 23:00


Mathematics, 01.10.2019 23:00


Social Studies, 01.10.2019 23:00

Mathematics, 01.10.2019 23:00




Mathematics, 01.10.2019 23:00



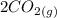 ⇄
⇄ 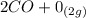
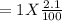

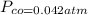
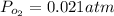
![Kp = \frac{[CO_{2} ]^{2} }{[CO]^{2} [O_{2} ] }](/tpl/images/0574/3678/cce9a.png)
![Kp = \frac{[0.958 ]^{2} }{[0.042]^{2} [0.021] }](/tpl/images/0574/3678/1e9e8.png)