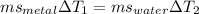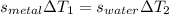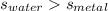Physics, 31.03.2020 20:31 tkdurocher
A 50-g chunk of a common metal at 80°C is placed into a calorimeter with 50 g of water at 50°C. At thermal equilibrium, which effect would be most likely?
Group of answer choices
The water will have changed temperature more than the metal.
The metal will have changed temperature more than the water.
The water will have gained more thermal energy than the metal lost.
The equilibrium temperature will be the average of 65°C.

Answers: 3


Another question on Physics

Physics, 22.06.2019 12:30
Compare the horizontal displacement of the two balls as they travel to the ground. what do you observe?
Answers: 1


Physics, 22.06.2019 19:00
The particle p starts from rest at point a at time t = 0 and changes its speed thereafter at a constant rate of 2.8g as it follows the horizontal path shown. determine the magnitude and direction of its total acceleration (a) just before point b, (b) just after point b, and (c) as it passes point c. state your directions relative to the x-axis shown (ccw positive) and choose the angle with the smallest magnitude.
Answers: 1

Physics, 22.06.2019 20:10
Atruck with 34-in.-diameter wheels is traveling at 55 mi/h. find the angular speed of the wheels in rad/min, *hint convert miles to inches & hours to minutes:
Answers: 2
You know the right answer?
A 50-g chunk of a common metal at 80°C is placed into a calorimeter with 50 g of water at 50°C. At t...
Questions


Mathematics, 12.05.2021 23:30



Mathematics, 12.05.2021 23:30

Mathematics, 12.05.2021 23:30



Physics, 12.05.2021 23:30





Mathematics, 12.05.2021 23:30

History, 12.05.2021 23:30




Chemistry, 12.05.2021 23:30