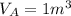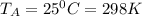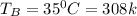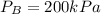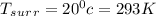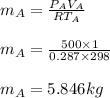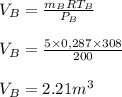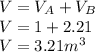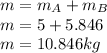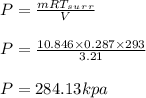Physics, 30.03.2020 17:57 twistedhyperboles
A 1-m3 tank containing air at 25 °C and 500 kPa is connected through a valve to another tank containing 5 kg of air at 35 °C and 200 kPa. Now the valve is opened, and the entire system is allowed to reach thermal equilibrium with the surroundings, which are at 20 °C. Determine the volume of the second tank and the final equilibrium pressure of air.

Answers: 2


Another question on Physics


Physics, 22.06.2019 04:40
Argon is adiabatically compressed from an initial volume of 16 liters to a final volume of 2 liters. by what factor do the following quantities change? do they increase or decrease? (a) the rms speed (b) the thermal energy of the gas (c) the molar specific heat cv (d) the pressure
Answers: 3

Physics, 22.06.2019 10:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3

Physics, 22.06.2019 12:00
You have a resistor and a capacitor of unknown values. first, you charge the capacitor and discharge it through the resistor. by monitoring the capacitor voltage on an oscilloscope, you see that the voltage decays to half its initial value in 2.70 miss . you then use the resistor and capacitor to make a low-pass filter. what is the crossover frequency fc?
Answers: 2
You know the right answer?
A 1-m3 tank containing air at 25 °C and 500 kPa is connected through a valve to another tank contain...
Questions

Social Studies, 22.12.2020 18:40


English, 22.12.2020 18:40

Mathematics, 22.12.2020 18:40

History, 22.12.2020 18:40


Health, 22.12.2020 18:40

Spanish, 22.12.2020 18:40




Social Studies, 22.12.2020 18:40

English, 22.12.2020 18:40

Mathematics, 22.12.2020 18:40

English, 22.12.2020 18:40


Mathematics, 22.12.2020 18:40


History, 22.12.2020 18:40

English, 22.12.2020 18:40