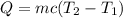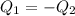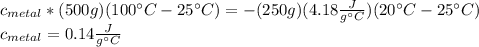Physics, 27.03.2020 17:07 camiee13dvivvj
En un experimento de calorimetría, 0.50 kg de un metal a 100°C se añaden a 0.50 kg de agua a 20°C en un vaso de calorímetro de aluminio, cuya masa es de 0.250 kg. A) Si un poco de agua salpica y sale del vaso al agregar el metal, el calor específico medido será 1) mayor, 2) igual o 3) menor que el valor calculado para el caso en que no se salpique agua. ¿Por qué? B) Si la temperatura final de la mezcla es de 25°C, y no se salpica agua, ¿qué calor específico tendrá el metal?

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:20
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3

Physics, 22.06.2019 11:00
Aperson walks first at a constant speed of 4.89 m/s along a straight line from point a to point b and then back along the line from b to a at a constant speed of 2.95 m/s. what is the average speed over the entire trip?
Answers: 1

Physics, 22.06.2019 15:10
Auniform crate c with mass mc is being transported to the left by a forklift with a constant speed v1. what is the magnitude of the angular momentum of the crate about point a, that is, the point of contact between the front tire of the forklift and the ground
Answers: 3

Physics, 22.06.2019 18:00
Gabby calls her cousin who lives in a different state and tells her to turn her radio to channel 98.7 so they can listen to their favorite song that is playing. her cousin turns her radio to 98.7, but does not hear the same song gabby hears. which most likely explains why?
Answers: 1
You know the right answer?
En un experimento de calorimetría, 0.50 kg de un metal a 100°C se añaden a 0.50 kg de agua a 20°C en...
Questions


Biology, 17.01.2021 14:00

Mathematics, 17.01.2021 14:00


Social Studies, 17.01.2021 14:00



History, 17.01.2021 14:00

Mathematics, 17.01.2021 14:00

Geography, 17.01.2021 14:00


Mathematics, 17.01.2021 14:00

English, 17.01.2021 14:00

Mathematics, 17.01.2021 14:00

History, 17.01.2021 14:00


History, 17.01.2021 14:00


Mathematics, 17.01.2021 14:00

English, 17.01.2021 14:00