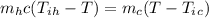A person pours 330 g of water at 55°C into an 855-g aluminum container with an initial temperature of 10°C. The specific heat of aluminum is 900 J/(kg・ΕK) and that of water is 4190 J/(kg・ΕK). What is the final temperature of the system, assuming no heat is exchanged with the surroundings?

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies. molten iron has a density of 7.0g/cm cubed. in its solid state, iron has a density of 8.0g/cm cubed.
Answers: 3

Physics, 22.06.2019 19:30
Emagnitude of the electrical force acting between a +2.4 × 10–8 c charge and a +1.8 × 10–6 c charge that are separated by 0.008 m is n, rounded to the tenths place.
Answers: 3

Physics, 22.06.2019 21:30
In the illustration, which two simple machines are being used to enable the student to reach the door? a. inclined plane and pulley b lever and wheel-and-axle c pulley and lever d wheel-and axle and inclined plane
Answers: 1

Physics, 23.06.2019 06:30
The center of the milky way most likely contains a. empty space. b. a red giant star. c. a globular cluster. d. a supermassive black hole.
Answers: 1
You know the right answer?
A person pours 330 g of water at 55°C into an 855-g aluminum container with an initial temperature o...
Questions



Business, 07.11.2019 07:31

Mathematics, 07.11.2019 07:31




Social Studies, 07.11.2019 07:31








History, 07.11.2019 07:31


History, 07.11.2019 07:31


Mathematics, 07.11.2019 07:31