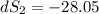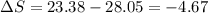One container holds 0.10 kg of water at 75 ∘C and is warmed to 95 ∘C by heating from contact with the other container. The other container, also holding 0.10 kg of water, cools from 35 ∘C to 15 ∘C. Specific heat of water is 4180 J/kg⋅∘C. Estimate the total change in entropy of two containers of water using the actual temperatures to determine the heat transferred to each container and the average temperatures to determine the change in entropy. Is this energy transfer process allowed by the first law of thermodynamics? Yes or No. is this energy transfer process allowed by the second law of thermodynamics? Yes or No

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 1

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1

Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 1

Physics, 22.06.2019 16:00
What part of the ear is names after tools, such as the hammer and the anvil?
Answers: 1
You know the right answer?
One container holds 0.10 kg of water at 75 ∘C and is warmed to 95 ∘C by heating from contact with th...
Questions

History, 02.07.2019 22:00

Mathematics, 02.07.2019 22:00

English, 02.07.2019 22:00

Biology, 02.07.2019 22:00



History, 02.07.2019 22:00



Mathematics, 02.07.2019 22:00

Mathematics, 02.07.2019 22:00





English, 02.07.2019 22:00


Mathematics, 02.07.2019 22:00


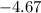
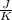
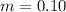 Kg
Kg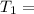 75°C
75°C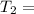 95°C
95°C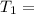 35°C
35°C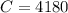
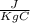
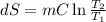
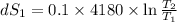
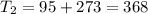 K,
K, 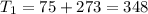 K
K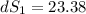
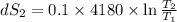
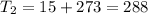 K,
K, 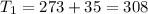 K
K