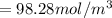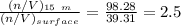Physics, 23.03.2020 16:54 Jamilia561
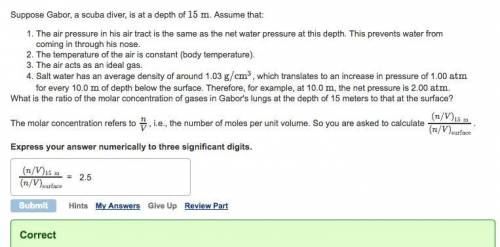
Suppose Gabor, a scuba diver, is at a depth of 15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3, which translates to an increase in pressure of 1.00 atm for every 10.0 m of depth below the surface. Therefore, for example, at 10.0 m, the net pressure is 2.00 atm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface

Answers: 1


Another question on Physics

Physics, 22.06.2019 01:20
Abanked road comer of radius 146 m is to be constructed. if the comer is designed for vehicles moving with a speed of 20.0 m/s, what should the banking angle be, in units of degrees?
Answers: 3

Physics, 22.06.2019 13:10
Aplane flying horizontally at an altitude of 1 mile and a speed of of 500mih passes directly over a radar station. find the rate at which the distance from the plane to the station is increasing when it is 2mi away from the station.
Answers: 1


Physics, 22.06.2019 17:40
Which component of the earth’s atmosphere is decreased due to photosynthesis?
Answers: 1
You know the right answer?
Suppose Gabor, a scuba diver, is at a depth of 15m. Assume that: The air pressure in his air tract i...
Questions



Mathematics, 16.07.2021 19:40

Mathematics, 16.07.2021 19:40

Mathematics, 16.07.2021 19:40







Mathematics, 16.07.2021 19:50

English, 16.07.2021 19:50

Mathematics, 16.07.2021 19:50

Mathematics, 16.07.2021 19:50

Mathematics, 16.07.2021 19:50

Mathematics, 16.07.2021 19:50

Mathematics, 16.07.2021 19:50



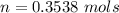
![P_d = [\frac{15m}{10m} ] (1 atm) + 1 atm](/tpl/images/0558/9055/c032e.png)
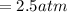
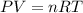
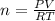
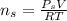
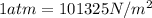 for
for 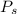 ,
,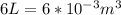 for volume , 8.314 J/mol. K for R , (37° +273) K for T into the equation
for volume , 8.314 J/mol. K for R , (37° +273) K for T into the equation 
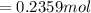
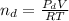
 are pressure and no of moles at the depth of the water
are pressure and no of moles at the depth of the water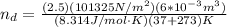
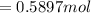
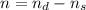
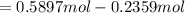

![[\frac{n}{V} ]_{surface} = \frac{0.2359mol}{6*10^-3m^3}](/tpl/images/0558/9055/08954.png)

![[\frac{n}{V} ]_{15m} = \frac{0.5897}{6*10^{-3}}](/tpl/images/0558/9055/d7097.png)