
Physics, 19.03.2020 08:00 hamptonjeleesa
A gas‑forming reaction produces 1.90 m 3 1.90 m3 of gas against a constant pressure of 179.0 kPa. 179.0 kPa. Calculate the work done by the gas in joules. Hint: be sure to convert the cubic meters to liters and kilopascals to atm. Look up the conversion factors!

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:00
In the first law of thermodynamics, triangle e=q-w, what does q stand for
Answers: 1


Physics, 22.06.2019 11:30
(1 point) match the differential equations and their vector valued function solutions. you may wish to multiply at least one solution out fully, to make sure that you know how to do it. you can get the other answers quickly by process of elimination and just multiply out one row element.
Answers: 2

Physics, 22.06.2019 15:20
Aphoton is absorbed by an electron that is in the n = 3 state of a hydrogen atom, causing the hydrogen atom to become ionized. very far away from the nucleus, the released electron has a velocity of 750,000 m/s. what was the wavelength of the absorbed photon?
Answers: 2
You know the right answer?
A gas‑forming reaction produces 1.90 m 3 1.90 m3 of gas against a constant pressure of 179.0 kPa. 17...
Questions


Business, 22.10.2020 17:01



Mathematics, 22.10.2020 17:01



Arts, 22.10.2020 17:01


Social Studies, 22.10.2020 17:01




Mathematics, 22.10.2020 17:01

Physics, 22.10.2020 17:01

English, 22.10.2020 17:01

Engineering, 22.10.2020 17:01


Mathematics, 22.10.2020 17:01

History, 22.10.2020 17:01

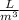 = 1900 L
= 1900 L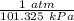 = 1.767 atm
= 1.767 atm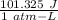 = 3.4 × 10⁵ J
= 3.4 × 10⁵ J

