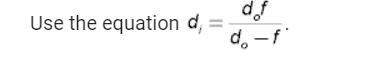Physics, 19.03.2020 00:03 jalenshayewilliams
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces carbon dioxide (CO2) and steam (H2O). The reaction can be described by the equation:
2C2H2 + 5O2 ➔ 4CO2 + 2H2O. How much mass of C2H2 is needed to react with 68.1 g of O2 to produce 75.0 g of CO2 and 15.35 g of steam?

Answers: 1


Another question on Physics


Physics, 22.06.2019 16:50
Which best describes the first law of thermodynamics as compared to the second law of thermodynamics? a. the first law describes how thermal energy is conserved but not the direction it moves. b. the first law describes the direction thermal energy moves but not how it is conserved. c. the first law describes how thermal energy can be created but not how it can be destroyed. d. the first law describes how thermal energy can be destroyed but not how it can be created.
Answers: 1

Physics, 23.06.2019 08:30
(asap) uneven heating of the earth's surface is due primarily to the position of the equator, north pole, sun, or moon?
Answers: 1

Physics, 23.06.2019 10:20
Q1=-2.00x10^-5 c, q2=+3.80x10^-6 c, q3=+5.30x10^-5 c. what is the electric potential energy for charge q2? between q1 and q2: 1.15m between q2 and q3: 2.88m
Answers: 3
You know the right answer?
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces carbon diox...
Questions

Mathematics, 06.04.2021 19:30



Chemistry, 06.04.2021 19:30

Chemistry, 06.04.2021 19:30

Mathematics, 06.04.2021 19:30

History, 06.04.2021 19:30

Mathematics, 06.04.2021 19:30

English, 06.04.2021 19:30


Mathematics, 06.04.2021 19:30

English, 06.04.2021 19:30

Chemistry, 06.04.2021 19:30

Mathematics, 06.04.2021 19:30

English, 06.04.2021 19:30

Mathematics, 06.04.2021 19:30

Social Studies, 06.04.2021 19:30

Social Studies, 06.04.2021 19:30


Biology, 06.04.2021 19:30

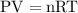 at a constant temperature,
at a constant temperature, 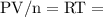 constant. That means,
constant. That means,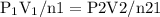
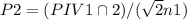
![P 2=(P 1 V 1 \cap 2) /(V 2 n 1)=\left[(155 a t m)^{*}(5.00 L)^{*}(2 m o l e s)\right] /\left[(25.00 L)^{*}(5 \text { moles }]\right]=124 \text { atm }](/tpl/images/0552/9219/932b9.png)