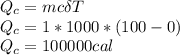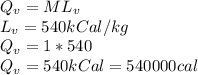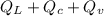The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:T, where c is the specific heat capacity of the substance. Forexample, forH20,c=1caljg'C",Andfora change of phase, the quantity of heat Q that changes the phase of a mass m is Q = ml., where L is the heat of fusion or heat of vaporization of the substance. For example, for H20, the heat offusion is 80 cal/g (or 80 kcaljkg) and the heat of vaporization is 540 cal/g (or 540 kcaljkg). Use these relationships to determine the number of calories to change (a) 1 kg ofO°C ice to O°C ice water, (b) 1 kg ofO°C ice water to 1 kg of 100°C boiling water, (c) 1 kg of 100°C boiling water to 1 kg of 100°C steam, and (d) 1 kg ofO°C ice to 1 kg of 100°C steam.

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:20
Awave is produced in a rope. the wave has a speed of 33 m/s and a frequency of 22 hz. what wavelength is produced?
Answers: 2

Physics, 23.06.2019 00:20
What efficiency of a car's engine when heat input is 200,000 joules and waste heat is 150,000 joules? a. 75% b. 35% c. 25% d. 85%
Answers: 2

Physics, 23.06.2019 03:50
Asimple electrochemical cell contains two electrodes? t or f
Answers: 2

Physics, 23.06.2019 08:10
Which of the following situations would produce an average velocity of zero? o a. a horse galloping in a field b. a round-trip to school and back o c. a trip, first to the moon, then onto mars o d. the criss-crossing path of a flying bug
Answers: 2
You know the right answer?
The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:...
Questions

Social Studies, 24.09.2020 21:01



Mathematics, 24.09.2020 21:01



Social Studies, 24.09.2020 21:01




Mathematics, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01





Mathematics, 24.09.2020 21:01

History, 24.09.2020 21:01

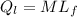 = 1 * 80
= 1 * 80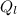 = 80 kCal = 80,000 cal
= 80 kCal = 80,000 cal