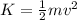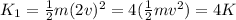Physics, 17.03.2020 05:14 devenairefp85xfg
A mass is pressed against (but is not attached to) an ideal horizontal spring on a frictionless horizontal surface. After being released from rest, the mass acquires a maximum speed v and a maximum kinetic energy K. If instead the mass initially compresses the spring twice as far:.
(a) its maximum speed will be 2v and its maximum kinetic energy will be 4K.
(b) its maximum speed will be 2v and its maximum kinetic energy will be √2K.
(c) its maximum speed will be 4v and its maximum kinetic energy will be 2K.
(d) its maximum speed will be 2v and its maximum kinetic energy will be 2K.
(e) its maximum speed will be v √2 and its maximum kinetic energy will be 2K.

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:30
Acoil formed by wrapping 80 turns of wire in the shape of a square is positioned in a magnetic field so that the normal to the plane of the coil makes an angle of 28.0° with the direction of the field. when the magnetic field is increased uniformly from 200 µt to 600 µt in 0.400 as, an emf of magnitude 80.0 mv is induced in the coil. what is the total length of the wire?
Answers: 3

Physics, 22.06.2019 03:30
Starting with only the balmer series light (visible light), how could we ensure that the solar panels generate a current that mark can use for his power station? a)by gradually increasing the brightness (amount) of light that we shine on it. b)by gradually increasing the frequency of the light we shine on it. c)by gradually increasing the wavelength of the light that we shine on it.
Answers: 3

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 07:50
Calculate the ratio of h+ ions to oh– ions at a ph = 6. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 6. are they the same? why or why not? record your explanation in table a. what is the concentration of h+ ions at a ph = 6? mol/l what is the concentration of oh– ions at a ph = 6? mol/l what is the ratio of h+ ions to oh– ions at a ph = 6? : 1
Answers: 1
You know the right answer?
A mass is pressed against (but is not attached to) an ideal horizontal spring on a frictionless hori...
Questions


Mathematics, 01.12.2021 20:40



Computers and Technology, 01.12.2021 20:40


Chemistry, 01.12.2021 20:40


Mathematics, 01.12.2021 20:40

English, 01.12.2021 20:40




Mathematics, 01.12.2021 20:40



Mathematics, 01.12.2021 20:40

Mathematics, 01.12.2021 20:40


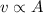 .
.