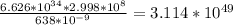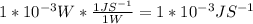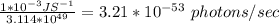A monochromatic red laser beam emitting 1 mW at a wavelength of 638 nm is incident on a silicon solar cell. Find the following: a. The number of photons per second incident on the cell b. The maximum possible efficiency of conversion of this laser c. beam to electricity

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:00
The crank oa rotates in the vertical plane with a constant clockwise angular velocity ω0 of 5.6 rad/s. for the position where oa is horizontal, calculate the force under the light roller b of the 10.6-kg slender bar ab.
Answers: 2

Physics, 22.06.2019 06:30
Organelles are 1. responsible for producing power for the cell 2. tiny structures in the cell that carry out the cell's activities 3. responsible for digestion in the cell 4. found outside of the membrane
Answers: 1

Physics, 22.06.2019 06:40
Alinearly polarized electromagnetic wave has an average intensity of 196 w/m^2. this wave is directed towards two ideal polarizers (in real polarizers, transmission is also effected by reflection and absorption). polarizer a is oriented with its transmission axis at an angle of θ_1=20.8∘ with the incident electric field. polarizer b has its axis at an angle of θ_2=63.0∘ with the incident electric field. what is the average intensity of the wave after it passes through polarizer a? what is the average intensity of the wave after it passes through polarizer b? suppose that the two polarizers a and b are interchanged. what would the average intensity be after passing through both polarizers?
Answers: 2

Physics, 22.06.2019 11:30
Considering only the earth's rotation, determine how much later the asteroid would have had to arrive to put the explosion above helsinki at longitude 25˚ e? this would have obliterated the city.
Answers: 1
You know the right answer?
A monochromatic red laser beam emitting 1 mW at a wavelength of 638 nm is incident on a silicon sola...
Questions


Mathematics, 24.06.2019 04:30


Health, 24.06.2019 04:30



Mathematics, 24.06.2019 04:30


Mathematics, 24.06.2019 04:30

Biology, 24.06.2019 04:30

French, 24.06.2019 04:30

Health, 24.06.2019 04:30


Health, 24.06.2019 04:30



History, 24.06.2019 04:30



Physics, 24.06.2019 04:30