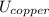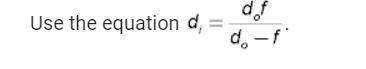Physics, 10.03.2020 04:37 eymurezgi1
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Assuming the surroundings to be at 20°C, determine the amount of work that could have been produced if the entire process were executed in a reversible manner.

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:00
If a substance is in the gas phase, which of qualities of the gas will stay constant? a: volume b: mass c: shape d: position of particles
Answers: 2

Physics, 22.06.2019 11:30
Which accounts for an increase in the temperature of a gas that is kept a constant volume
Answers: 1

Physics, 22.06.2019 11:50
Select all that applywhat are some basic resources a family is expected to provide for children? educationclothesspending
Answers: 2

Physics, 22.06.2019 18:30
Asmall grinding wheel has a moment of inertia of 4.0×10−5 kg⋅m2 . what net torque must be applied to the wheel for its angular acceleration to be 150 rad/s2 ?
Answers: 3
You know the right answer?
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake a...
Questions

Mathematics, 18.06.2020 17:57


Engineering, 18.06.2020 17:57







Mathematics, 18.06.2020 17:57






Mathematics, 18.06.2020 17:57


Arts, 18.06.2020 17:57

English, 18.06.2020 17:57

 = Δ
= Δ + Δ
+ Δ