
0.3-L glass of water at 20C is to be cooled with ice to 5C. Determine how much ice needs to be added to the water, in grams, if the ice is at (a) 0C and (b) –20C. Also (c) determine how much water would be needed if the cooling is to be done with cold water at 0C. The melting temperature and the heat of fusion of ice at atmospheric pressure are 0C and 333.7 kJ/kg, respectively, and the density of water is 1 kg/L.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
The us government wants to allocate billions of dollars in the next 10 years to assure our future energy security. the funds will be spread among a variety of possible energy resources. where do you think the government should put the greatest support: solar energy, wind energy, clean coal, oil exploration, gas exploration, or a combination of sources? are there other efforts that should be explored? support your position with cited information for both questions.
Answers: 2

Physics, 21.06.2019 23:20
Which quantities are scalars? choose all that apply a.distance b.speed c. acceleration d.velocity
Answers: 2

Physics, 22.06.2019 11:20
How to tell if a molecule is polar or nonpolar with electronegativity
Answers: 2

You know the right answer?
0.3-L glass of water at 20C is to be cooled with ice to 5C. Determine how much ice needs to be add...
Questions



Chemistry, 17.10.2019 23:20



Health, 17.10.2019 23:20



Mathematics, 17.10.2019 23:20






Mathematics, 17.10.2019 23:20





Mathematics, 17.10.2019 23:20


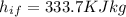
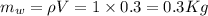 .
.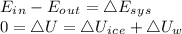
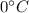 is defined as:
is defined as:![[mc(0-T_1)+mh_i_f+mc(T_2-0)]_i_c_e+[mc(T_2-T_1)]_w=0\\\\m_i_c_e[0+333.7+418\times(5-0)]+0.3\times4.18\times(5-20)=0\\m_i_c_e=0.0546Kg=54.6g](/tpl/images/0534/1119/67777.png)
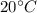 is defined as:
is defined as:![[mc(0-T_1)+mh_i_f+mc(T_2-0)]_i_c_e+[mc(T_2-T_1)]_w=0\\\\m_i_c_e[2.11\times(0-(20))+333.7+4.18\times(5-0)]+0.3\times4.18\times(5-20)=0\\\\m_i_c_e=0.0487Kg=48.7g](/tpl/images/0534/1119/47737.png)
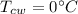
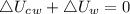
![[mc(T_2-T_1)]_c_w+[mc(T_2-T_1)]_w=0\\m_c_w\times4.18\times(5-0)+0.3\times4.18\times(5-20)\\m_c_w=0.9kg=900g](/tpl/images/0534/1119/fa4d4.png)


