
High-altitude mountain climbers do not eat snow, but always melt it first with a stove. To see why, calculate the energy absorbed from a climber's body under the following conditions. The specific heat of ice is 2100 J/kg⋅C∘, the latent heat of fusion is 333 kJ/kg, the specific heat of water is 4186 J/kg⋅C∘

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:50
What is the type of the reaction below? bacl2 + h2so4 → 2 hci + baso4 double replacement synthesis single replacement decomposition
Answers: 1

Physics, 22.06.2019 05:30
An object weighs 40n in air, weighs 20n when submerged in water and 30n when submerged in a liquid of unknown density. what is the density of the liquid?
Answers: 2

Physics, 22.06.2019 06:30
The mini-refrigerator fire was most likely caused by what type of wiring?
Answers: 2

Physics, 22.06.2019 12:30
Write a full page that sumerizes thermodynamics it’s from the website visionlearnig
Answers: 1
You know the right answer?
High-altitude mountain climbers do not eat snow, but always melt it first with a stove. To see why,...
Questions

Mathematics, 22.07.2021 21:20

Social Studies, 22.07.2021 21:20

Mathematics, 22.07.2021 21:20




English, 22.07.2021 21:20

Mathematics, 22.07.2021 21:20

English, 22.07.2021 21:20

Chemistry, 22.07.2021 21:20

Mathematics, 22.07.2021 21:20




History, 22.07.2021 21:20

Geography, 22.07.2021 21:20

English, 22.07.2021 21:20

Computers and Technology, 22.07.2021 21:20

Geography, 22.07.2021 21:20

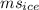 ΔT = (1.0)(2.100×10³)(15) = 3.150×
ΔT = (1.0)(2.100×10³)(15) = 3.150×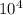 J
J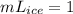 ×
×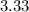 ×
×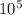 = 3.33×
= 3.33×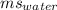 ΔT = 1×4.186×
ΔT = 1×4.186×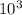 ×37 = 1.548×
×37 = 1.548×

