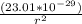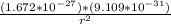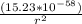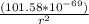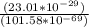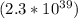Physics, 02.03.2020 21:03 almighty196
Determine the ratio of the electrostatic force to the gravitational force between a proton and an electron, FE/FG. Note: k = 8.99 × 109 N-m2/C2; G = 6.672 × 10–11 N-m2/kg2; me = 9.109 ×

Answers: 3


Another question on Physics

Physics, 21.06.2019 23:10
6–55 refrigerant-134a enters the condenser of a residential heat pump at 800 kpa and 358c at a rate of 0.018 kg/s and leaves at 800 kpa as a saturated liquid. if the compressor consumes 1.2 kw of power, determine (a) the cop of the heat pump and (b) the rate of heat absorption from the outside air.
Answers: 2

Physics, 22.06.2019 10:00
Need people build a dam to create a reservoir that supplies water a nearby city needs. describe two ways this action will likely affect the water cycle in the local environment. (5 points) worth 20 points
Answers: 1

Physics, 22.06.2019 18:30
Ablock of mass m slides on a horizontal frictionless table with an initial speed v0 . it then compresses a spring of force constant k and is brought to rest. the acceleration of gravity is 9.8 m/s2. how much is the spring compressed x from its natural length? 1) x = v0*sqrt(k/(mg)) 2) x=v0*sqrt(m/k) 3) x=v0*((mk)/g) 4) x=v0*sqrt(k/m) 5) x=v0*(m/kg) 6) x=v0*sqrt((mg)/k) 7) x=(v0)^2/(2g) 8) x=v0*(k/(mg)) 9) x=(v0)^2/(2m) 10) x=v0*((mg)/k)
Answers: 3

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3
You know the right answer?
Determine the ratio of the electrostatic force to the gravitational force between a proton and an el...
Questions





Mathematics, 08.04.2020 04:20

English, 08.04.2020 04:20











Mathematics, 08.04.2020 04:20



Mathematics, 08.04.2020 04:20

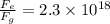
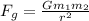
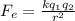
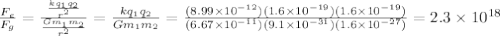
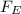 ) between two particles is given as;
) between two particles is given as;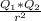 --------------------(i)
--------------------(i)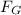 ) between two particles is given as;
) between two particles is given as;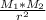 --------------------(ii)
--------------------(ii)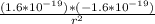
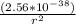 [negative sign can be discarded]
[negative sign can be discarded]