Physics, 27.02.2020 03:26 smarty5187
In performing the Grignard reaction under the conditions of Experiment 16, if you were to use 121 mg of benzophenone (182.21 g/mol), 18 mg of magnesium (24.30 g/mol), and 76 microliters of bromobenzene (157.02 g/mol, 1.50 g/mL), calculate the theoretical yield of triphenylmethanol (260.33 g/mol), in mg. Benzophenone is the limiting reactant. Enter your answer as digits only, no units, using the proper number of significant figures.

Answers: 1


Another question on Physics


Physics, 22.06.2019 14:00
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 22.06.2019 14:20
4r-134a enters the condenser of a residential heat pump at 800 kpa and 50°c at a rate of 0.022 kg/s and leaves at 750 kpa subcooled by 3°c. the refrigerant enters the compressor at 200 kpa superheated by 4°c determine (a) the isentropic efficiency of the compressor, (b) the rate of heat supplied to the heated room, and (c) the cop of the heat pump. also, determine (d) the cop and rate of heat supplied to the heated room if this heat pump operated on the ideal vapor-compression cycle between the pressure limits of 200 and 800 kpa. (0.757, 4.37 kw, 5.12, 6.18, 3.91 kw)
Answers: 3

Physics, 22.06.2019 17:30
Patricia is trying to compare the average rainfall of new york to that of arizona. a comparison between these two states for the months of july through september would best be measured in
Answers: 3
You know the right answer?
In performing the Grignard reaction under the conditions of Experiment 16, if you were to use 121 mg...
Questions

Mathematics, 23.09.2020 14:01

Computers and Technology, 23.09.2020 14:01







Health, 23.09.2020 14:01



Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

 .....(1)
.....(1)

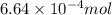 of bromobenzene will produce =
of bromobenzene will produce =  moles of triphenylmethanol
moles of triphenylmethanol 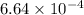 moles
moles