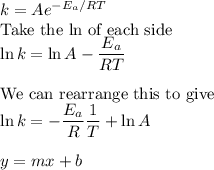Physics, 25.02.2020 23:32 deanazilyiah
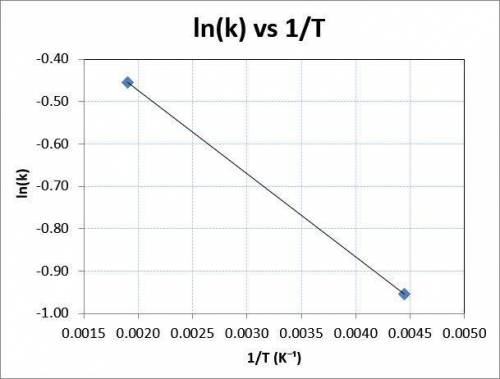
Consider the reaction data. A ⟶ products T ( K ) k ( s − 1 ) 225 0.385 525 0.635 What two points should be plotted to graphically determine the activation energy of this reaction? To avoid rounding errors, use at least three significant figures in all values. x 1 = y 1 = x 2 = y 2 = Determine the rise, run, and slope of the line formed by these points. rise: run: slope: What is the activation energy of this reaction? E a = J / mol

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:00
Mike walks 100 meter north, then walks 30 meters south. after this, he walks another 10 meters north. what is the magnitude of his total displacement during this walk,in meters?
Answers: 2

Physics, 22.06.2019 04:40
Steam enters an adiabatic diffuser at 150 kpa and 1208c with a velocity of 550 m/s. determine the minimum velocity that the steam can have at the outlet when the outlet pressure is 300 kpa.
Answers: 3


Physics, 22.06.2019 11:10
Assume this car is driven off a cliff . how many arrows of force need to be drawn in the free body diagram? assume no air resistance -five -one -three -four
Answers: 1
You know the right answer?
Consider the reaction data. A ⟶ products T ( K ) k ( s − 1 ) 225 0.385 525 0.635 What two points sho...
Questions


Mathematics, 14.12.2021 19:20




Mathematics, 14.12.2021 19:20




Social Studies, 14.12.2021 19:20

History, 14.12.2021 19:20


Engineering, 14.12.2021 19:20

Spanish, 14.12.2021 19:20

Mathematics, 14.12.2021 19:20

Mathematics, 14.12.2021 19:20

Mathematics, 14.12.2021 19:20

History, 14.12.2021 19:20