
Calculate the energy in the form of heat (in kJ) required to change 75.0 g of liquid water at 27.0 °C to ice at –20.0 °C. Assume that no energy in the form of heat is transferred to the environment. (Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: ice = 2.06 J/g·K, liquid water = 4.184 J/g·K)

Answers: 3


Another question on Physics

Physics, 22.06.2019 17:30
Aparticle moves in a circle 1.50 m in radius . through what angle in radians does it rotate if it moves through an arc length of 2.50m? what is the angle in degrees?
Answers: 1

Physics, 22.06.2019 19:30
The nuclear potential that binds protons and neutrons in the nucleus of an atom is often approximated by a square well. imagine a proton conned in an innite square well of length 105 nm, a typical nuclear diameter. calculate the wavelength and energy associated with the photon that is emitted when the proton undergoes a transition from the rst excited state (n 2) to the ground state (n 1). in what region of the electromagnetic spectrum does this wavelength belong?
Answers: 1

Physics, 22.06.2019 21:30
Complete the sentence to describe the law of conservation of energy. the law of conservation of energy states that energy cannot be created
Answers: 3

Physics, 23.06.2019 00:30
What is the coldest temperature ever recorded in san antonio?
Answers: 1
You know the right answer?
Calculate the energy in the form of heat (in kJ) required to change 75.0 g of liquid water at 27.0 °...
Questions

SAT, 07.12.2021 03:20






Mathematics, 07.12.2021 03:20

SAT, 07.12.2021 03:20



Mathematics, 07.12.2021 03:20

English, 07.12.2021 03:20




SAT, 07.12.2021 03:20

Mathematics, 07.12.2021 03:20



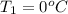
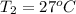
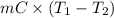
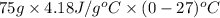
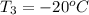 = (20 + 273) K = 293 K and specific heat of ice is 2.108 kJ/kg K.
= (20 + 273) K = 293 K and specific heat of ice is 2.108 kJ/kg K. 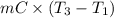
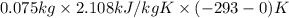
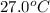 to ice at
to ice at 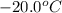 is -37.86 kJ.
is -37.86 kJ.


