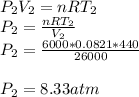Physics, 22.02.2020 02:49 nathanbrockdac
A sealed 26-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K. The gas is heated to a final temperature of 440 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol·K = 0.0821 L·atm/mol·K. The final pressure of the gas is closest to
A) 0.31
B) 0.34
C) 0.33
D) 0.36
E) 0.29

Answers: 2


Another question on Physics

Physics, 22.06.2019 08:30
How is sound energy produced a. vibrating objects b. objects being heated c. temperature differences d. stationary objects
Answers: 2

Physics, 22.06.2019 17:30
You can watch static electricity in action by rubbing an inflated balloon against your hair. your hair will actually stand on its end! it does this because each hair becomes charged, and they all repel one another.
Answers: 3

Physics, 22.06.2019 22:00
Which of these is the best way to manage a natural resource
Answers: 3

Physics, 23.06.2019 01:00
The bubbles in a carbonated soft drink are produced when carbonic acid decomposes to form carbon dioxide and water. in a closed system, this equilibrium exists as why does a carbonated soft drink lose carbonation when the container is left open? a.water evaporates, which favors the formation of h2co3. b.pressure decreases, which favors the formation of h2co3. c.the open container warms up, which causes more gas to escape. d.co2is removed, which favors the formation of h2co3.
Answers: 2
You know the right answer?
A sealed 26-m3 tank is filled with 6000 moles of oxygen gas (O2) at an initial temperature of 270 K....
Questions

Chemistry, 10.12.2020 21:50


Business, 10.12.2020 21:50


Mathematics, 10.12.2020 21:50

Health, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50

Biology, 10.12.2020 21:50


Mathematics, 10.12.2020 21:50

Physics, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50



Spanish, 10.12.2020 21:50


Spanish, 10.12.2020 21:50

Mathematics, 10.12.2020 21:50