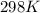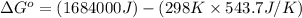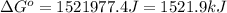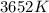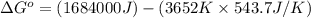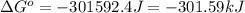The chemical reaction that causes iron to corrode in air is given by
4Fe + 3O2→2Fe2O3 in which...

Physics, 21.02.2020 23:38 ellycleland16
The chemical reaction that causes iron to corrode in air is given by
4Fe + 3O2→2Fe2O3 in which at 298 K
ΔHrxn= 1684 kJ
ΔSrxn= 543.7 J/K
Part A. What is the standard Gibbs free energy for this reaction? Assume the commonly used standard reference temperature of 298 K.
Part B. What is the Gibbs free energy for this reaction at 3652 K? Assume that Delta H and Delta S do not change with temperature.

Answers: 1


Another question on Physics

Physics, 22.06.2019 12:30
Hydrogen atoms are excited by a laser to the =4n=4 state and then allowed to emit. what is the maximum number of distinct emission spectral lines (lines of different wavelengths) that can be observed from this system? 8 6 2 7 4 5 1 3 calculate the wavelength of the 4⟶14⟶1 transition. =λ=
Answers: 2

Physics, 22.06.2019 14:30
What distance does a car travel as its speed changes from 0 to 20 m/s in 17 s at constant acceleration?
Answers: 1

Physics, 22.06.2019 14:30
Alandscaper is shopping for landscaping materials. she wants to use materials through which water flows easily. which materials should she choose? check all that apply. clay gravel granite rocks with cracks loosely packed soil
Answers: 2

Physics, 22.06.2019 14:40
Glass has a hardness that is in the middle of the hardness scale. what is the hardness of glass?
Answers: 1
You know the right answer?
Questions


Mathematics, 21.03.2020 11:43

Mathematics, 21.03.2020 11:43

Biology, 21.03.2020 11:43



Business, 21.03.2020 11:43


Mathematics, 21.03.2020 11:43

Chemistry, 21.03.2020 11:43




History, 21.03.2020 11:44


Mathematics, 21.03.2020 11:44




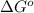 at 298 K is, 1521.9 kJ
at 298 K is, 1521.9 kJ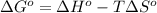
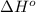 = standard enthalpy = 1684 kJ = 1684000 J
= standard enthalpy = 1684 kJ = 1684000 J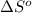 = standard entropy = 543.7 J/K
= standard entropy = 543.7 J/K