Physics, 21.02.2020 23:05 ciralove2004
Consider the elementary gas-phase reversible reaction A 3C Pure A enters at a temperature of 400 K and a pressure of 10 atm. At this temperature, KC 0.25(mol/dm3)2. Calculate the equilibrium conversion for each of the following situations:

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:30
Will give brainliest! jay rides his 2.0-kg skateboard. he is moving at speed 5.8 m/s when he pushes off the board and continues to move forward in the air at 5.4 m/s. the board now goes forward at 13 m/s.a. determine jay’s mass.b. determine the change in the internal energy of the system during this process.(express your answer to two significant figures and include the appropriate units.)
Answers: 1

Physics, 22.06.2019 10:30
Find the magnetic field a distance r from the center of a long wire that has radius a and carries a uniform current per unit area j in the positive z direction.
Answers: 2

Physics, 22.06.2019 16:30
Which magnetic property best describes a magnet’s ability to act at a distance? magnets are dipolar. magnets attract only certain objects. magnets have magnetic fields. magnets can transfer their properties to certain materials.
Answers: 1

Physics, 22.06.2019 19:20
The dipole moment of the water molecule (h2o) is 6.17x10^-30 c.m. consider a water molecule located at the origin whose dipole moment p points in the +x-direction. a chlorine ion ( of charge-1.60x10^-19c , is located at x=3.00x10^-9m . assume that is much larger than the separation d between the charges in the dipole, so that the approximate expression for the electric field along the dipole axis can be used. a) find the magnitude of the electric force that the water molecule exerts on the chlorine ion. b) what is the direction of the electric force. -x-direction or +x-direction c) is this force attractive or repulsive?
Answers: 1
You know the right answer?
Consider the elementary gas-phase reversible reaction A 3C Pure A enters at a temperature of 400 K a...
Questions


Geography, 17.01.2020 00:31









Arts, 17.01.2020 00:31




Social Studies, 17.01.2020 00:31





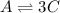



![\% = [0.304mol/liter-0.1845mol/liter]/(0.304mol/liter)\times 100](/tpl/images/0519/7722/78e3b.png)