
Alice and Tom dive from an overhang into the lake below. Tom simply drops straight down from the edge, but Alice takes a running start and jumps with an initial horizontal velocity of 25 m/s. Neither person experiences any significant air resistance. Just as they reach the lake below
A) the speed of Alice is larger than that of Tom.
B) the splashdown speed of Alice is larger than that of Tom.
C) they will both have the same speed.
D) the speed of Tom will always be 9.8 m/s larger than that of Alice.
E) the speed of Alice will always be 25 m/s larger than that of Tom.

Answers: 3


Another question on Physics

Physics, 21.06.2019 17:20
Research the neutrino using the internet and report your findings. in a nuclear decay, the nucleus of an atom splits apart. larger particles can be detected and their masses and velocities can be recorded. explain how the existence and properties of the neutrino could be predicted using the conservation laws.
Answers: 3

Physics, 21.06.2019 20:30
Water at room temperature of 20.0°c is poured into an aluminum cylinder which has graduation markings etched on the inside. the reading in the graduations is 300.0 cc. the cylinder with the water in it is then immersed in a constant temperature bath at a temperature of 100°c. what is the reading for the level of water on the graduations of the cylinder after the water and the cylinder reach thermal equilibrium with the bath? the volume coefficient of expansion of water is 2.07 x 10^-4 k-1, and the linear coefficient of expansion of aluminum is 23.0 x 10^-6 k-1. a) 305.0 cc b) 304.0 cc c) 303.5 cc d) 303.3 cc e) 304.5 cc
Answers: 3

Physics, 22.06.2019 05:30
Acombination reaction is when two or more combine to form one product. a decomposition reaction is when a substance breaks down into two or more simpler substances in a chemical reaction.
Answers: 1

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3
You know the right answer?
Alice and Tom dive from an overhang into the lake below. Tom simply drops straight down from the edg...
Questions

Mathematics, 24.04.2020 18:53

English, 24.04.2020 18:53



Social Studies, 24.04.2020 18:53

Computers and Technology, 24.04.2020 18:53


Spanish, 24.04.2020 18:53




Biology, 24.04.2020 18:54





Mathematics, 24.04.2020 18:54


Chemistry, 24.04.2020 18:54

Biology, 24.04.2020 18:54

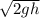 for both .
for both . 

