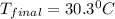An ice cube at 0.00 ∘C with a mass of 21.5 g is placed into 500.0 g of water, initially at 31.0 ∘C, in an insulated container. Part A Assuming that no heat is lost to the surroundings, what is the temperature of the entire water sample after all of the ice has melted?

Answers: 3


Another question on Physics

Physics, 22.06.2019 12:00
If two students are running down the hall toward each other, trying to get to class, and they have the same mass and acceleration, what will happen when they collide? will their forces cancel out or will each one experience a reaction?
Answers: 1


Physics, 22.06.2019 19:00
Which of the following list the divisions of the geologic time scale in order from longest to shortest
Answers: 1

You know the right answer?
An ice cube at 0.00 ∘C with a mass of 21.5 g is placed into 500.0 g of water, initially at 31.0 ∘C,...
Questions


Mathematics, 18.08.2021 07:50


English, 18.08.2021 07:50


Mathematics, 18.08.2021 07:50





Mathematics, 18.08.2021 07:50


Mathematics, 18.08.2021 07:50


Mathematics, 18.08.2021 07:50

Mathematics, 18.08.2021 07:50

Physics, 18.08.2021 07:50

Mathematics, 18.08.2021 07:50


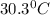
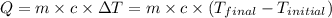 .......(1)
.......(1)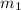 = mass of ice = 21.5 g
= mass of ice = 21.5 g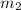 = mass of water = 500.0 g
= mass of water = 500.0 g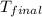 = final temperature = ?
= final temperature = ?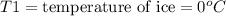
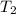 = temperature of water =
= temperature of water =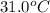
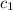 = specific heat of ice=
= specific heat of ice= 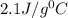
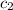 = specific heat of water =
= specific heat of water = 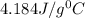
![21.5\times 2.1\times (T_{final}-0)=-[500.0\times 4.184\times (T_{final}-31.0)]](/tpl/images/0513/4270/003f3.png)