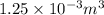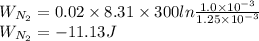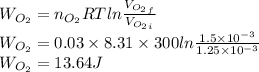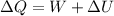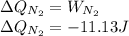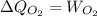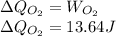Physics, 15.02.2020 04:00 lizzytyler4725
52. A metallic container of fixed volume of 2.5 × 10−3 m3 immersed in a large tank of temperature 27 °C contains two compartments separated by a freely movable wall. Initially, the wall is kept in place by a stopper so that there are 0.02 mol of the nitrogen gas on one side and 0.03 mol of the oxygen gas on the other side, each occupying half the volume. When the stopper is removed, the wall moves and comes to a final position. The movement of the wall is controlled so that the wall moves in infinitesimal quasi-static steps. (a) Find the final volumes of the two sides assuming the ideal gas behavior for the two gases. (b) How much work does each gas do on the other? (c) What is the change in the internal energy of each gas? (d) Find the amount of heat that enters or leaves each gas.

Answers: 3


Another question on Physics

Physics, 22.06.2019 02:00
In which situation has the independent variable for the experiment been changed? check all that apply. changed from counting the number of duckweed at day 0 and day 14 to counting them every day changed from testing duckweed growth in beakers to testing duckweed growth in large outdoor tanks changed from testing the effect of ph on duckweed growth to testing the effect of light levels on duckweed growth changed from testing the effect of acid ph on duckweed growth to testing the effect of basic ph on duckweed growth
Answers: 1

Physics, 22.06.2019 23:50
What is part of a line has one endpoint and continues in one direction?
Answers: 1


You know the right answer?
52. A metallic container of fixed volume of 2.5 × 10−3 m3 immersed in a large tank of temperature 27...
Questions

Mathematics, 22.06.2019 17:00


History, 22.06.2019 17:00


Biology, 22.06.2019 17:00










Business, 22.06.2019 17:00

Chemistry, 22.06.2019 17:00




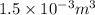 whereas that of nitrogen is
whereas that of nitrogen is 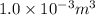
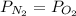
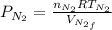
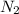 which is given as 0.02 mol.
which is given as 0.02 mol.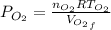
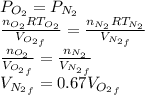
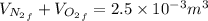
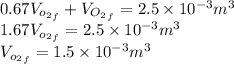
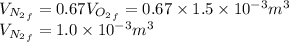
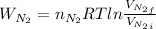
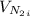 is half of the total volume i.e
is half of the total volume i.e