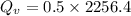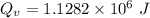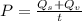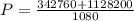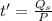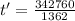Physics, 15.02.2020 03:44 aleshachrishon42
Water is boiled at sea level in a coffeemaker equipped with an immersion-type electric heating element. The coffee maker contains 1 L of water when full. Once boiling starts, it is observed that half of the water in the coffeemaker evaporates in 18 min. Determine the power rating of the electric heating element immersed in water. Also, determine how long it will take for this heater to raise the temperature of 1 L of cold water from 18°C to the boiling temperature. The enthalpy of vaporization of water at the saturation temperature of 100ºC iss hfg = 2256.4 kJ/kg. At an average temperature of (100 + 18)/2 = 59ºC, the specific heat of water is c = 4.18 kJ/kg·ºC and the density is about 1 kg/L.

Answers: 3


Another question on Physics


Physics, 22.06.2019 20:40
Newtons view of arrangement of the universe. don't copy from anywhere.
Answers: 3


Physics, 22.06.2019 22:20
Which symbol in the first law of thermodynamics represents the sum of the chemical and thermal energy stored in atoms and molecules? a. q b.w c.v d.u
Answers: 3
You know the right answer?
Water is boiled at sea level in a coffeemaker equipped with an immersion-type electric heating eleme...
Questions






English, 22.07.2021 01:00






Mathematics, 22.07.2021 01:00

Mathematics, 22.07.2021 01:00

Mathematics, 22.07.2021 01:00


Mathematics, 22.07.2021 01:00


Mathematics, 22.07.2021 01:00


Mathematics, 22.07.2021 01:00

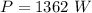
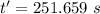 is time required to heat to boiling point form initial temperature.
is time required to heat to boiling point form initial temperature.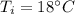
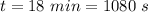
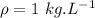
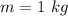
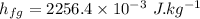
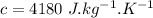
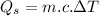
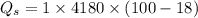
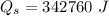
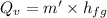
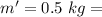 mass of water vaporized due to boiling
mass of water vaporized due to boiling