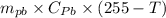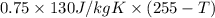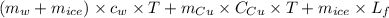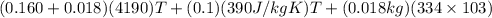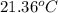A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal equilibrium at atmospheric pressure.
Part A
If 0.750kg of lead at a temperature of 255 c is dropped into the calorimeter can, what is the final temperature? Assume that no heat is lost to the surroundings

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:20
Three charge are arranged as shown in the diagram. the magnitude of the net electrical force acting on the +6 uc charge, rounded to the tenths place, is .
Answers: 1

Physics, 22.06.2019 11:50
The electric field between square plates of a parallel-plate capacitor has magnitude e. the potential across the plates is maintained with constant voltage by a battery as they are pulled apart to twice their original separation, which is small compared to the dimensions of the plates. the magnitude of the electric field between the plates is now equal to a)e b)e/4 c)e/2 d)4e e)2e
Answers: 1

Physics, 22.06.2019 14:30
Alandscaper is shopping for landscaping materials. she wants to use materials through which water flows easily. which materials should she choose? check all that apply. clay gravel granite rocks with cracks loosely packed soil
Answers: 2

Physics, 22.06.2019 17:00
Using proper grammar, spelling, and punctuation, write at least one 5 sentence paragraph describing 3 ways we use the elements of the electromagnetic spectrum (ems) in our everyday lives.
Answers: 2
You know the right answer?
A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal e...
Questions


Chemistry, 11.06.2021 22:00




Mathematics, 11.06.2021 22:00


Mathematics, 11.06.2021 22:00



Mathematics, 11.06.2021 22:00



Mathematics, 11.06.2021 22:00


Mathematics, 11.06.2021 22:00

Mathematics, 11.06.2021 22:00


Mathematics, 11.06.2021 22:00

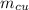 ) = 0.1 kg
) = 0.1 kg
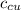 ) = 390 J/kg K
) = 390 J/kg K
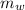 ) = 0.160 kg
) = 0.160 kg
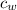 ) = 4190 J/kg K
) = 4190 J/kg K
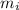 ) = 0.018 kg
) = 0.018 kg
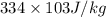
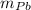 ) = 0.75 kg
) = 0.75 kg
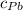 ) = 130 J/kg K
) = 130 J/kg K