
Physics, 13.02.2020 23:54 TheOverlordOfWhales
A) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3 closed container. If the gas goes through an isochoric process to twice the initial temperature, what is the new pressure of the gas in Pa?
b) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3closed container. If the gas goes through an isothermal process to 3.6 m3, what is the new pressure of the gas in Pa?
c) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3 closed container. If the gas goes through an isobaric process to 3.6 m3, what is the new temperature of the gas in Kelvin?

Answers: 1


Another question on Physics

Physics, 22.06.2019 07:00
Oxygen and hydrogen gas are at the same temperature t.what is the ratio of kinetic energies of oxygen molecule and hydrogen molecule if oxygen is 16 times heavier than hydrogen.
Answers: 3

Physics, 22.06.2019 11:20
If the radius of curvature of the cornea is 0.75 cm when the eye is focusing on an object 36.0 cm from the cornea vertex and the indexes of refraction are as described before, what is the distance from the cornea vertex to the retina? express your answer to two significant
Answers: 3

Physics, 22.06.2019 17:20
You charge a parallel-plate capacitor, remove it from the battery, and prevent the wires connected to the plates from touching each other. when you increase the plate separation, what happens to the following quantities?
Answers: 1

Physics, 23.06.2019 05:00
The inverse square law applies to: intensity of sound gravitational force intensity of light electric force magnetic force
Answers: 1
You know the right answer?
A) Consider 1.3 moles of an ideal gas at an initial temperature of 400 K and in a 1.2 m3 closed cont...
Questions


Social Studies, 01.12.2021 20:40


Business, 01.12.2021 20:40

Mathematics, 01.12.2021 20:40




Computers and Technology, 01.12.2021 20:40




Mathematics, 01.12.2021 20:40

History, 01.12.2021 20:40

Biology, 01.12.2021 20:40

Biology, 01.12.2021 20:40


Physics, 01.12.2021 20:40

Mathematics, 01.12.2021 20:40

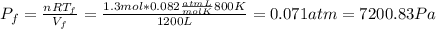
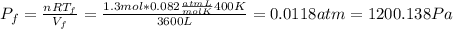

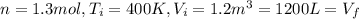
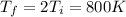 ,
, 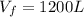 n = 1.3 mol and we can find the final pressure like this:
n = 1.3 mol and we can find the final pressure like this: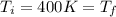 since the process is isothermal
since the process is isothermal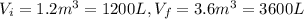
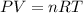
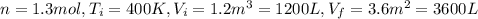
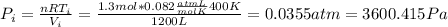
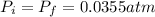 and we can find the final temperature like this:
and we can find the final temperature like this:

