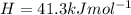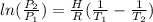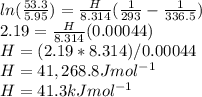Answers: 2


Another question on Physics

Physics, 21.06.2019 15:20
Two large, flat, horizontally oriented plates are parallel to each other, a distance d apart. half way betwween the two plates the electric field has magnitude e. if the separation of the plates is reduced to d/2 what is the magnitude of the electric field half way between the plates?
Answers: 2

Physics, 21.06.2019 21:30
Apendulum has a mass of 1.5 kg and starts at a height of 0.4 m. if it is released from rest, how fast is it going when it reaches the lowest point of its path? acceleration due to gravity is g = 9.8 m/s2. a. 2.8 m/s b. 0 m/s c. 5.9 m/s d. 4.3 m/s
Answers: 1

Physics, 22.06.2019 04:00
All the simple machines make work easier to do by changing the or of a force. a. size; type b. work; type c. size; direction d. type; direction
Answers: 2

Physics, 22.06.2019 05:30
An object weighs 40n in air, weighs 20n when submerged in water and 30n when submerged in a liquid of unknown density. what is the density of the liquid?
Answers: 2
You know the right answer?
The vapor pressure of ethanol at 293 K is 5.95 kPa and at 336.5 K it is 53.3 kPa. Calculate the enth...
Questions

English, 28.06.2019 11:30



Mathematics, 28.06.2019 11:30





Health, 28.06.2019 11:30


Health, 28.06.2019 11:30




Health, 28.06.2019 11:30


History, 28.06.2019 11:30



Chemistry, 28.06.2019 11:30