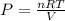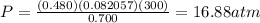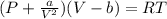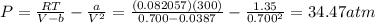Assume that you have 0.480 mol of N2 in a volume of 0.700 L at 300 K .
1. Calculate the...

Physics, 11.02.2020 05:28 eweqwoewoji
Assume that you have 0.480 mol of N2 in a volume of 0.700 L at 300 K .
1. Calculate the pressure in atmospheres using the ideal gas law.
2. Calculate the pressure in atmospheres using the van der Waals equation. For N2 , a=1.35 (L2⋅atm)/mol2 , and b=0.0387 L/mol

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:20
Two friends are having a conversation. anna says a satellite in orbit is in freefall because the satellite keeps falling toward earth. tom says a satellite in orbit is not in freefall because the acceleration due to gravity is not 9.80 m/2 . who do you agree with and why?
Answers: 1

Physics, 22.06.2019 14:00
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 23.06.2019 10:00
Acar tire rotates at a constant angular velocity of 3.5 rotations during a time interval of 0.75 seconds. what is the angular speed of the tire? in advance for any !
Answers: 2

Physics, 23.06.2019 10:00
No receiver is named? transitive passive or intrasitive complete
Answers: 2
You know the right answer?
Questions



Computers and Technology, 30.11.2020 23:30

English, 30.11.2020 23:30

Mathematics, 30.11.2020 23:30




Physics, 30.11.2020 23:30





Mathematics, 30.11.2020 23:30






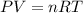 (1)
(1)