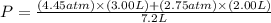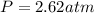Physics, 29.01.2020 05:44 elexiafloyd
You have a 3.00-liter container filled with n₂ at 25°c and 4.45 atm pressure connected to a 2.00-liter container filled with ar at 25°c and 2.75 atm pressure. a stopcock connecting the containers is opened and the gases are allowed to equilibrate between the two containers. what is the final pressure in the two containers if the temperature remains at 25°c? assume ideal behavior.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:30
After a big snowfall, you take your favorite rocket-powered sled out to a wide field. the field is 195 m across, and you know that your sled accelerates at a rate of 3.65 m/s2 when the rocket is on. how much time will it take the sled to cross the field starting from rest, assuming the rocket is on the whole time?
Answers: 1

Physics, 22.06.2019 02:30
If a refrigerator is a heat pump that follows the first law of thermodynamics, how much heat was removed from food inside of the refrigerator if it released 380j of energy to the room?
Answers: 1

Physics, 22.06.2019 14:30
Which of these is constant for all types of electromagnetic radiation in a vacuum? select one: a. wavelength b. frequency c. photon energy d. amplitude e. velocity
Answers: 3

Physics, 22.06.2019 17:00
Sawyer is studying diffraction. he draws a diagram of a plane wave to show how light waves travel. which best describes sawyer’s error? the wave fronts should be perpendicular to the direction in which the waves move. the arrow showing the direction of movement of the waves should be pointing to the left. the arrow showing the direction of movement of the waves should be pointing downward. the wave fronts should be both parallel and perpendicular to the direction in which the waves move.
Answers: 3
You know the right answer?
You have a 3.00-liter container filled with n₂ at 25°c and 4.45 atm pressure connected to a 2.00-lit...
Questions

History, 31.05.2020 00:00

Mathematics, 31.05.2020 00:00




Advanced Placement (AP), 31.05.2020 00:00

Mathematics, 31.05.2020 00:00









History, 31.05.2020 00:00

Mathematics, 31.05.2020 00:00

History, 31.05.2020 00:00


Mathematics, 31.05.2020 00:00

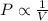
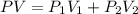
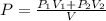
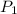 = pressure of N₂ gas = 4.45 atm
= pressure of N₂ gas = 4.45 atm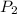 = pressure of Ar gas = 2.75 atm
= pressure of Ar gas = 2.75 atm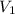 = volume of N₂ gas = 3.00 L
= volume of N₂ gas = 3.00 L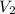 = volume of Ar gas = 2.00 L
= volume of Ar gas = 2.00 L